Abstract
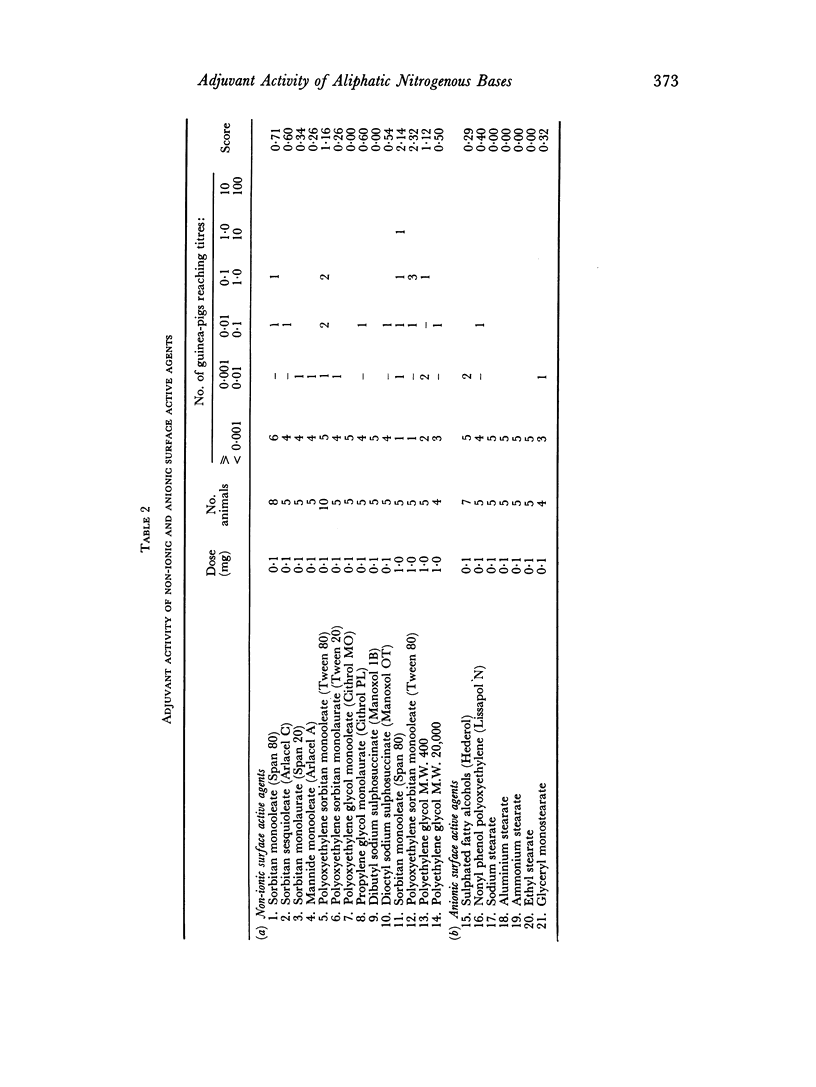
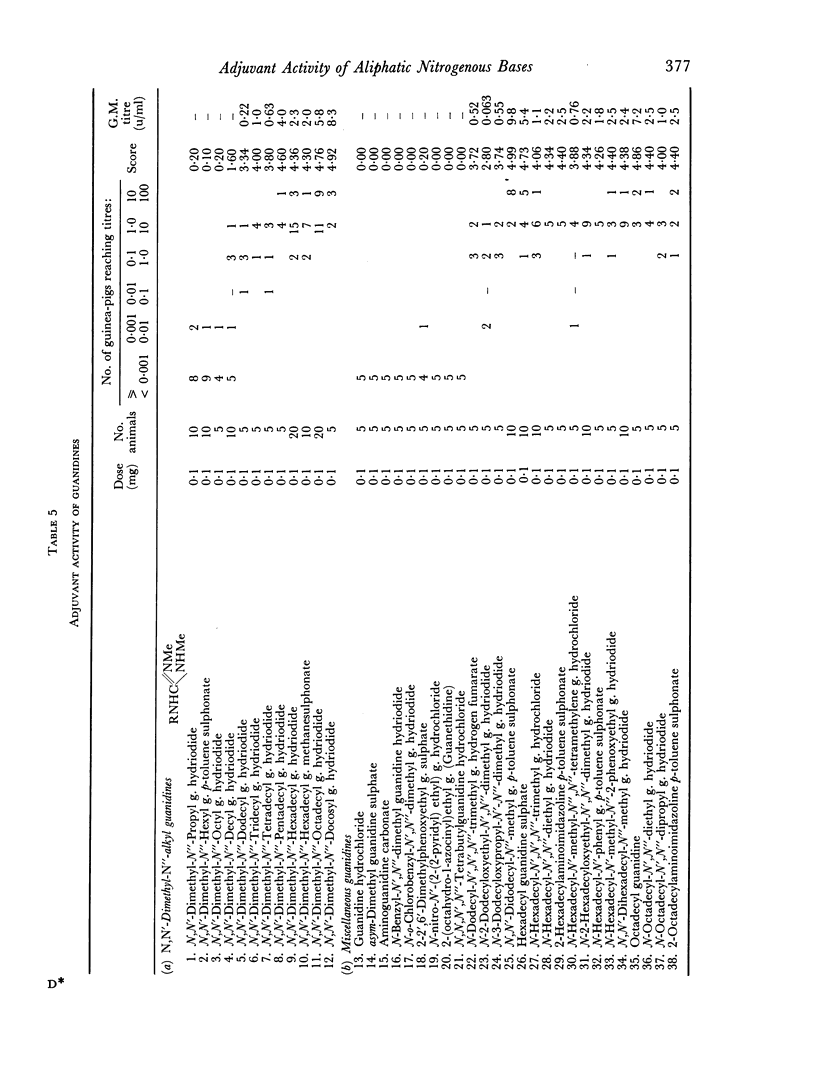
By the use of diphtheria toxoid in guinea-pigs, high adjuvant activity has been found in a number of aliphatic nitrogenous bases including amines, quaternary ammonium compounds, guanidines, benzamidines and thiouroniums. Activity appears to depend on a combination of basicity and a long aliphatic chain of twelve or more carbon atoms. Such adjuvants tend to be haemolytic, and cause damage to tissue culture monolayers. It is suggested that their activity is connected with their surface activity and hence their ability to alter cell membranes, but that the basicity plays a further as yet undetermined role.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMIES C. R. The use of topically formed calcium alginate as a depot substance in active immunisation. J Pathol Bacteriol. 1959 Apr;77(2):435–442. doi: 10.1002/path.1700770214. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., REES K. R., SHOTLANDER V. Penetration of lipid films by compounds preventing liver necrosis in rats. Nature. 1962 Feb 24;193:754–756. doi: 10.1038/193754a0. [DOI] [PubMed] [Google Scholar]
- CARR I. Appositional phagocytosis. J Pathol Bacteriol. 1962 Apr;83:443–448. [PubMed] [Google Scholar]
- DOURMASHKIN R. R., DOUGHERTY R. M., HARRIS R. J. Electron microscopic observations on Rous sarcoma virus and cell membranes. Nature. 1962 Jun 23;194:1116–1119. doi: 10.1038/1941116a0. [DOI] [PubMed] [Google Scholar]
- HARLEY J. D., MARGOLIS J. Haemolytic activity of colloidal silica. Nature. 1961 Mar 25;189:1010–1011. doi: 10.1038/1891010a0. [DOI] [PubMed] [Google Scholar]
- HUSSON F., LUZZATI V. Structure of red-cell ghosts and the effect of saponin treatment. Nature. 1963 Feb 23;197:822–822. doi: 10.1038/197822a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., NEAL R. A., GALL D. PROTECTIVE EFFECT OF KILLED TRYPANOSOME VACCINES WITH INCORPORATED ADJUVANTS. Nature. 1963 Oct 5;200:83–83. doi: 10.1038/200083a0. [DOI] [PubMed] [Google Scholar]
- MUIR A. R. An electron microscopic demonstration of a surface pattern on the plasma membrane of sectioned intestinal epithelium after saponin treatment. Nature. 1962 Sep 8;195:1023–1024. doi: 10.1038/1951023b0. [DOI] [PubMed] [Google Scholar]
- NOLL H., YOUNGNER J. S. Virus-lipid interactions. II. The mechanism of adsorption of lipophilic viruses to water-insoluble polar lipids. Virology. 1959 Jul;8(3):319–343. doi: 10.1016/0042-6822(59)90033-9. [DOI] [PubMed] [Google Scholar]
- RICHOU R., JENSEN R., BELIN C. RECHERCHES SUR LA SAPONINE, SUBSTANCE ADJUVANTE ET STIMULANTE DE L'IMMUNIT'E. I. Rev Immunol Ther Antimicrob. 1964 Jan-Mar;28:49–62. [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BARDAWIL W. A. 'Piggy-back' phagocytosis. Nature. 1962 Apr 21;194:255–256. doi: 10.1038/194255a0. [DOI] [PubMed] [Google Scholar]
- WEISS D. W., DUBOS R. J. Antituberculous immunity induced by methanol extracts of tubercle bacilli; its enhancement by adjuvants. J Exp Med. 1956 Jan 1;103(1):73–85. doi: 10.1084/jem.103.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODHOUR A. F., JENSEN K. E., WARREN J. Antibody response to influenza vaccines combined with hexadecylamine. Proc Soc Exp Biol Med. 1960 Jan;103:200–204. doi: 10.3181/00379727-103-25459. [DOI] [PubMed] [Google Scholar]
- YOUNGNER J. S., AXELROD V. ANTIGENICITY OF LIPID-ADSORBED DIPHTHERIA AND TETANUS TOXOIDS. J Immunol. 1964 Jun;92:879–884. [PubMed] [Google Scholar]