Abstract
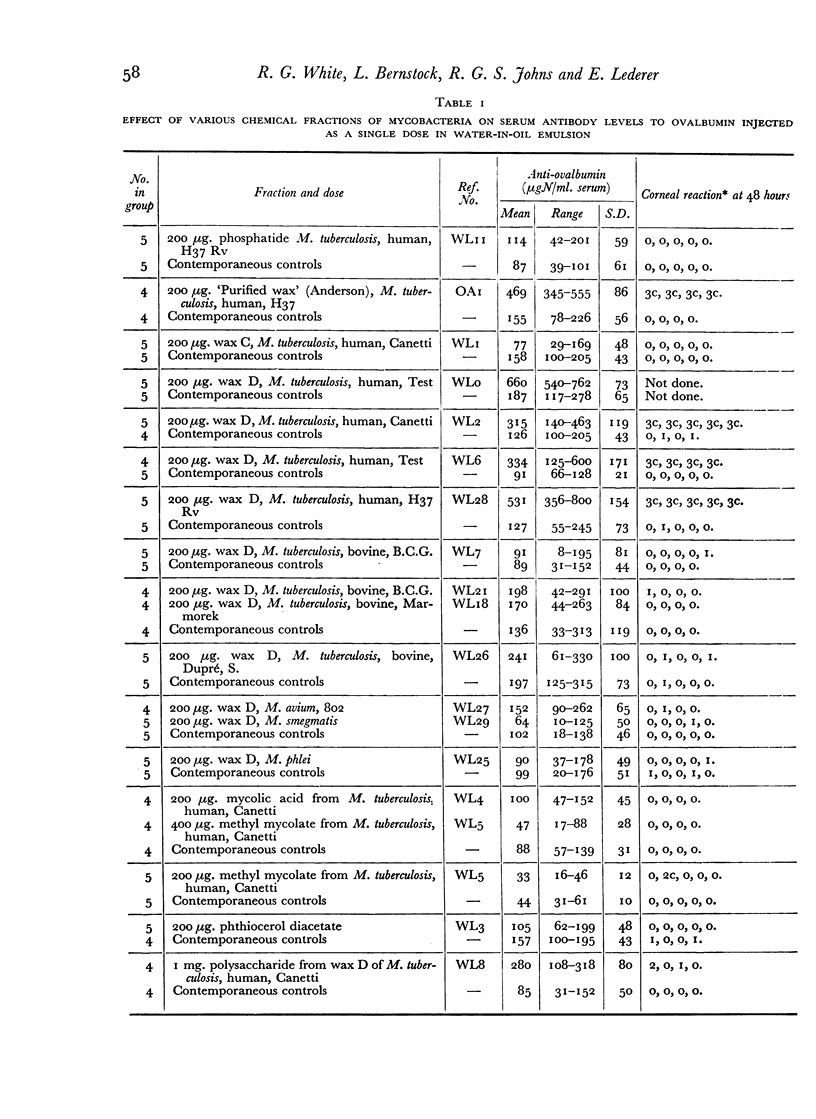
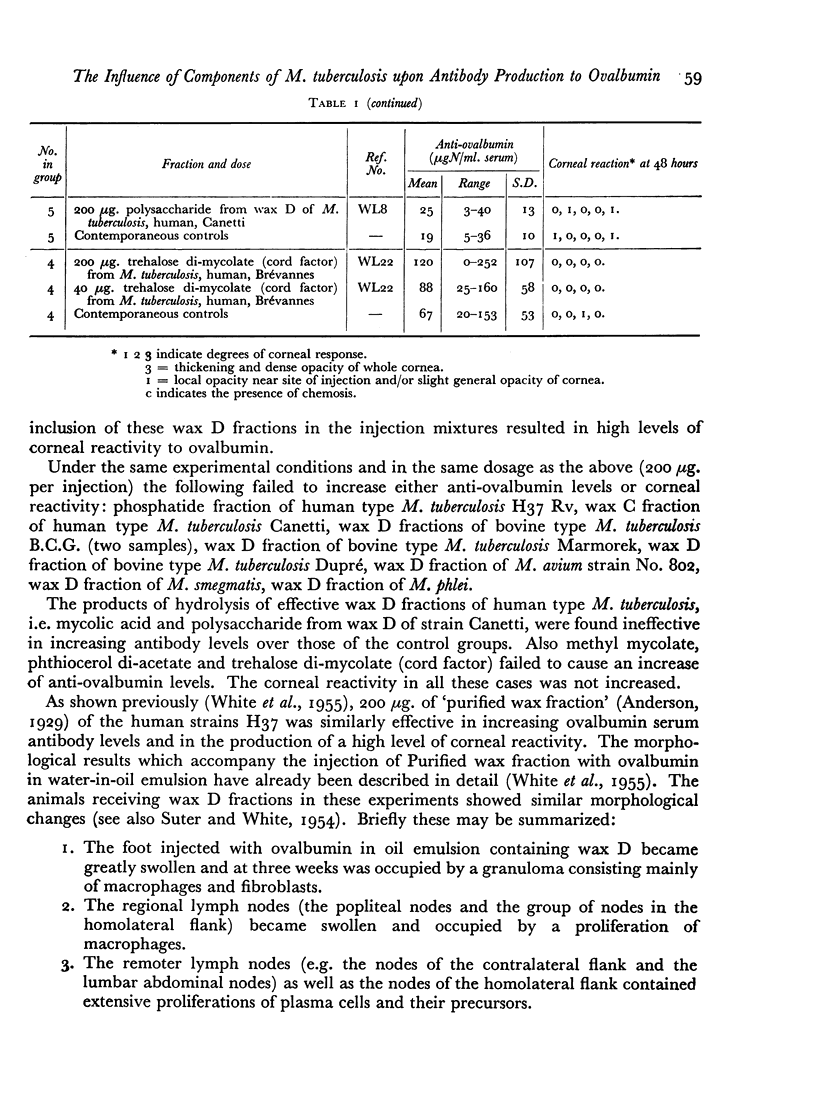
The inclusion of wax D fractions of the strains Test, Canetti and H37 Rv of human type M. tuberculosis in water-in-oil emulsions containing ovalbumin produced an increase in serum anti-ovalbumin levels measured at three weeks following a single injection into guinea-pigs, as compared with the levels of antibody in control animals which received ovalbumin emulsions lacking these fractions.
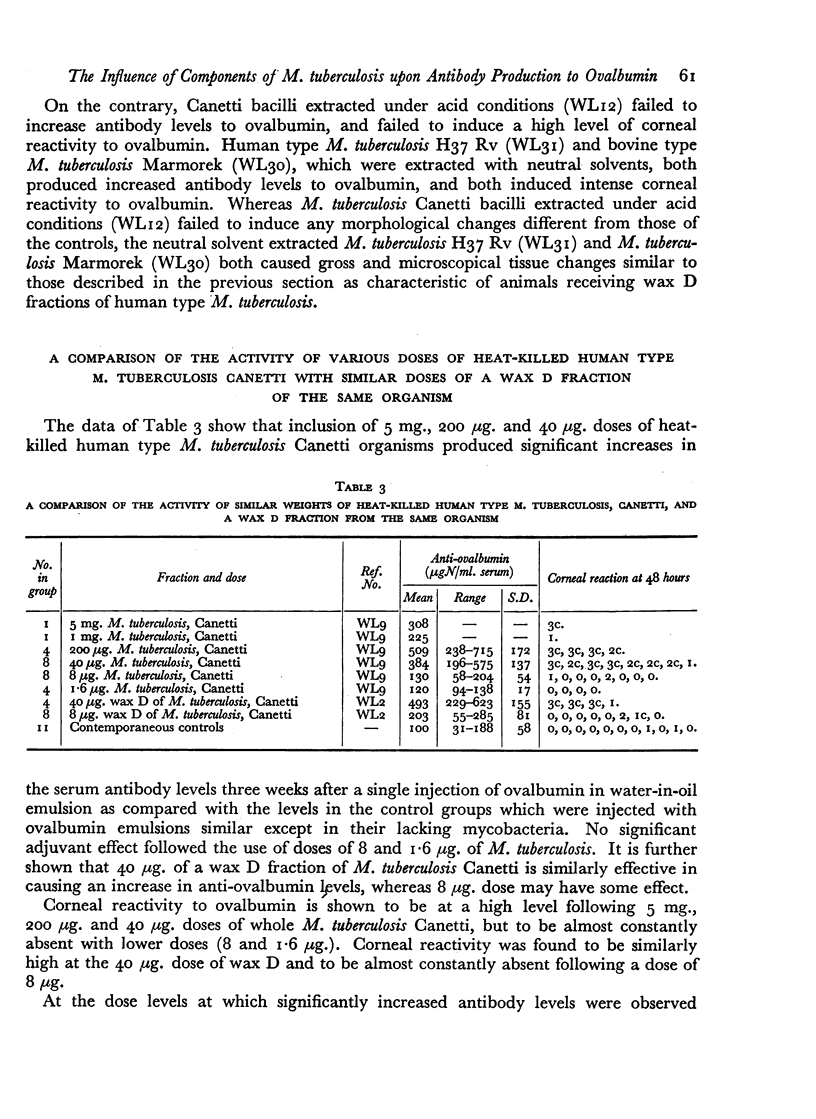
When this activity is determined at five-fold dose differences with equal weights of whole heat-killed M. tuberculosis Canetti and a wax D fraction of this organism both showed the same order of activity within the levels of accuracy of the trial, and both were active at doses of 40 μg. and above.
The following fractions from Mycobacteria were found to be inactive in increasing serum anti-ovalbumin under these conditions: phosphatide of M. tuberculosis H37 Rv, wax C of M. tuberculosis Canetti, wax D of M. avium strain No. 802, wax D of M. phlei and M. smegmatis, phthiocerol di-acetate, trehalose di-mycolate (cord factor), mycolic acid, methyl mycolate and polysaccharide from wax D of M. tuberculosis Canetti.
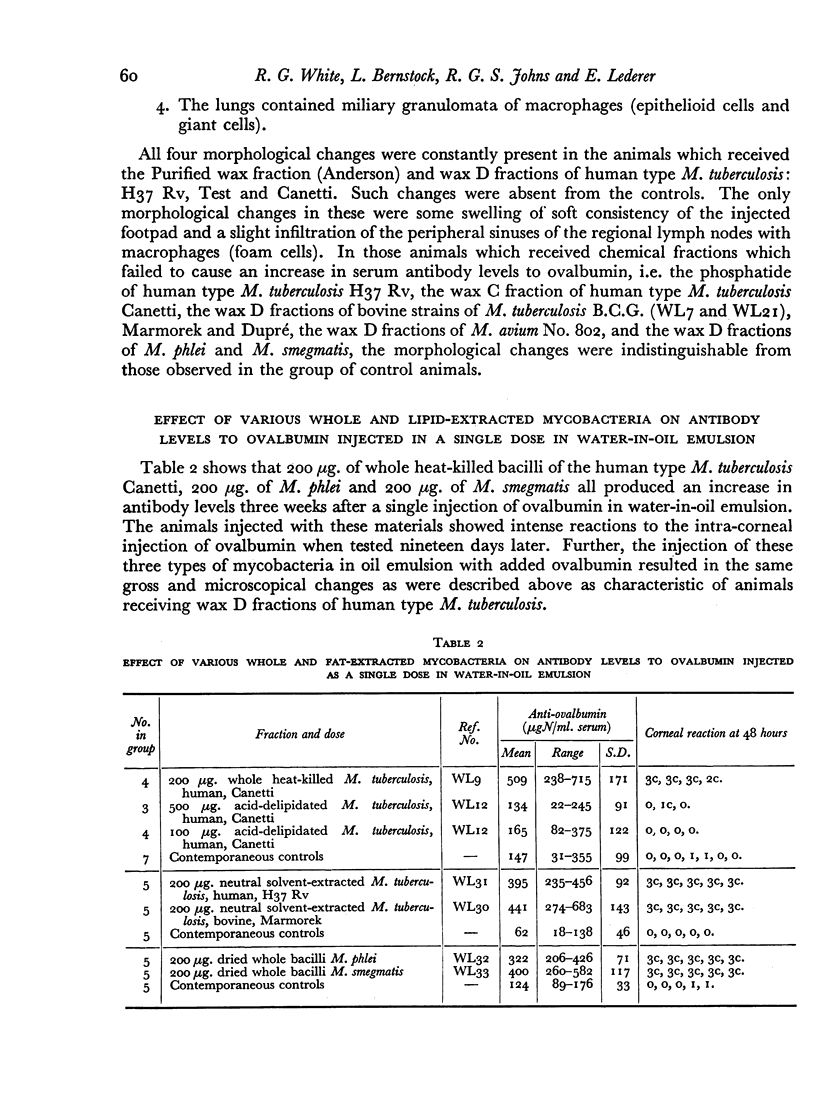
Dried bacilli from three Mycobacteria: M. tuberculosis B.C.G., M. smegmatis, M. phlei and delipidated M. tuberculosis of the bovine type Marmorek were found to be active in increasing serum anti-ovalbumin levels.
The activity of the wax D fractions of human types and the inactivity of the corresponding fractions of bovine, avian, and saprophytic types of Mycobacteria suggest that the adjuvant activity of the former is specifically due to the presence of the amino acids alanine, glutamic acid and α,ε-diaminopimelic acid they contain; these three amino acids, forming a peptide linked to the polysaccharide which is esterified with mycolic acid, seem to be necessary for the adjuvant activity observed in our experiments; hydrolysis destroys the activity of these wax D fractions.
The activity of the delipidated bacterial residues of human and bovine M. tuberculosis, as well as avian and saprophytic Mycobacteria, can be explained by the probable presence in these materials of a similar insoluble compound containing the three amino acids (alanine, glutamic acid and α,ε-diaminopimelic acid) linked to a polysaccharide which is esterified with mycolic acid.
Data are given of the morphological changes in guinea-pigs which constantly followed the injection of active bacilli or fractions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W. F. ENHANCEMENT OF THE IMMUNIZING CAPACITY OF INFLUENZA VIRUS VACCINES WITH ADJUVANTS. Science. 1944 Jun 2;99(2579):453–454. doi: 10.1126/science.99.2579.453. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A., Cannan R. K. The hydrogen ion dissociation curve of the crystalline albumin of the hen's egg. Biochem J. 1936 Feb;30(2):227–234. doi: 10.1042/bj0300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- RAFFEL S. Chemical factors involved in the induction of infectious allergy. Experientia. 1950 Nov 15;6(11):410–419. doi: 10.1007/BF02150118. [DOI] [PubMed] [Google Scholar]
- SUTER E., WHITE R. G. The response of the reticulo-endothelial system to the injection of the purified wax and the lipopolysaccharide of tubercle bacilli; a histologic and an immunologic study. Am Rev Tuberc. 1954 Nov;70(5):793–805. doi: 10.1164/art.1954.70.5.793. [DOI] [PubMed] [Google Scholar]
- WARD R., RADER D., LIPTON M. M., FREUND J. Formation of neutralizing antibody in monkeys injected with poliomyelitis virus and adjuvants. Proc Soc Exp Biol Med. 1950 Jul;74(3):536–539. doi: 10.3181/00379727-74-17964. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., COONS A. H., CONNOLLY J. M. Studies on antibody production. IV. The role of a wax fraction of Mycobacterium tuberculosis in adjuvant emulsions on the production of antibody to egg albumin. J Exp Med. 1955 Jul 1;102(1):83–104. doi: 10.1084/jem.102.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]


