Abstract
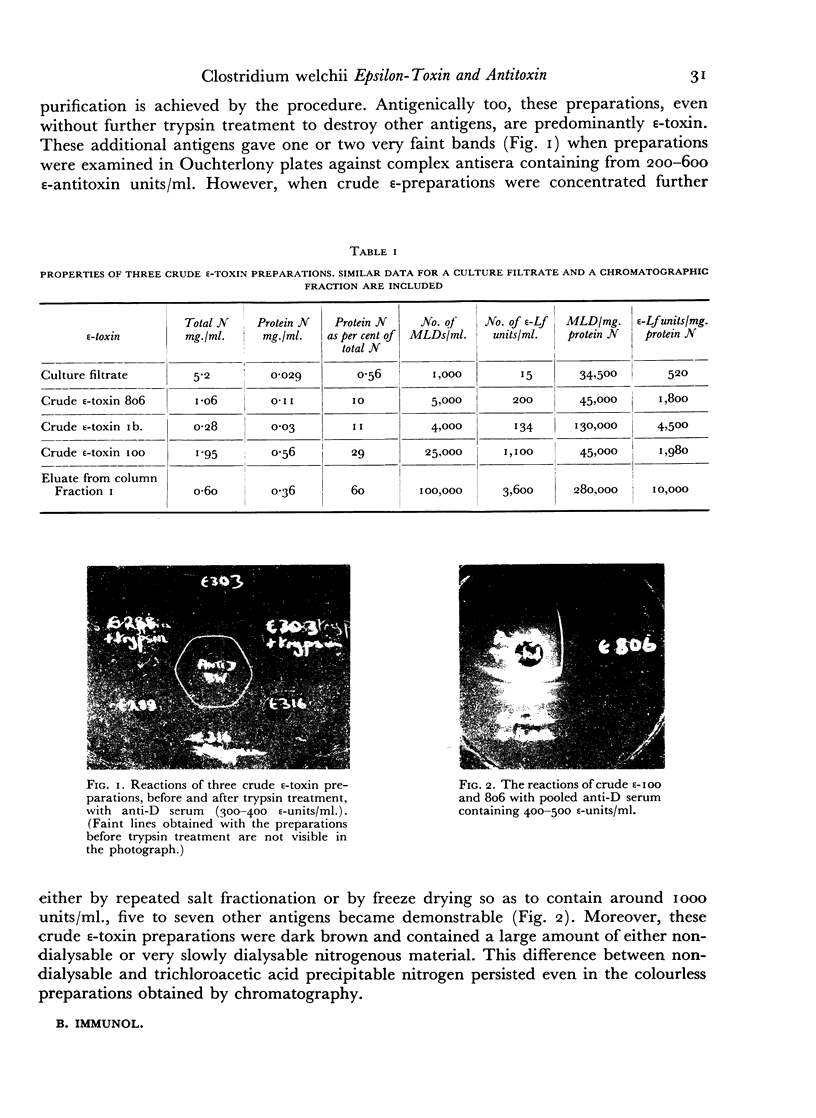
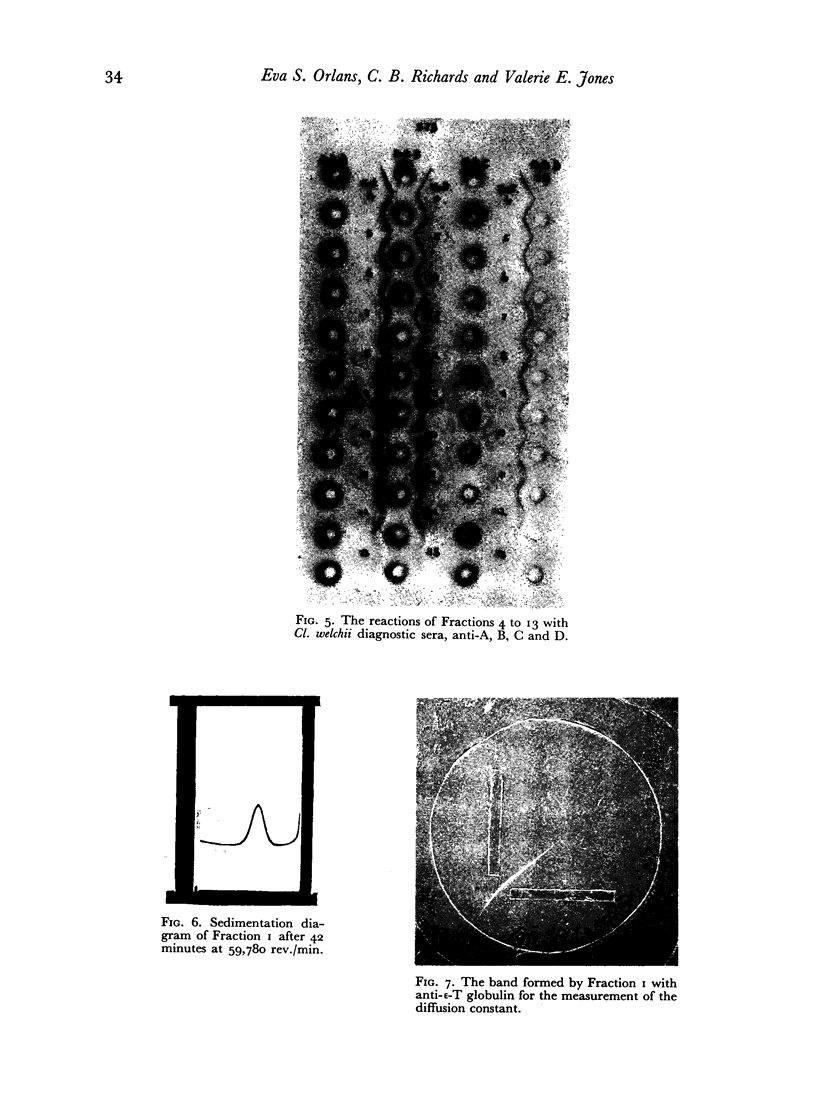
The ε-toxin of Cl. welchii Type D has been isolated in an immunologically and electrophoretically homogeneous state, by elution from DEAE cellulose. Such a preparation, containing 10,000 Lf/mg. N, sedimented as a single symmetrical peak in the ultracentrifuge, but still contained some non-protein N. The molecular weight of ε-toxin was estimated as 38,000 and its toxicity in mice as 280,000 MLD/mg. protein N. Its combination with horse antitoxin was studied by neutralization tests in vivo, by the flocculation reaction (optimum proportions), by quantitative precipitation and by double diffusion in agar gel. The immunological and chemical relationships between the non-toxic ε-precursor and the active toxin derived from it by tryptic (or other enzymic) hydrolysis, and toxoid made from either material by treatment with β-propiolactone, were also investigated.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., HUMPHREY J. H. Estimation of the size of antigens by gel diffusion methods. Nature. 1959 Jun 6;183(4675):1590–1592. doi: 10.1038/1831590a0. [DOI] [PubMed] [Google Scholar]
- BIDWELL E. Proteolytic enzymes of Clostridium welchii. Biochem J. 1950 May;46(5):589–598. doi: 10.1042/bj0460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTIN P. Coexistence de complexes précipitants et non-précipitants dans la réaction entre la sérum-albumine humaine et les immunsérums spécifiques de chevaux; déductions quantitatives et conséquences théoriques. Bull Soc Chim Biol (Paris) 1955;37(9-10):977–994. [PubMed] [Google Scholar]
- BURTIN P. Etude des sérums de chevaux immunisés avec la sérumalbumine humaine. Bull Soc Chim Biol (Paris) 1954;36(2-3):335–345. [PubMed] [Google Scholar]
- Bard R. C., McClung L. S. Biochemical Properties of the Toxins of Clostridium novyi and Clostridium hemolyticum. J Bacteriol. 1948 Nov;56(5):665–670. doi: 10.1128/jb.56.5.665-670.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT S. D. The crystallization and serological differentiation of a streptococcal proteinase and its precursor. J Exp Med. 1950 Sep;92(3):201–218. doi: 10.1084/jem.92.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S., SIMPSON M. E. The chromatography of growth hormone on cellulose derivatives. J Biol Chem. 1956 Jun;220(2):939–949. [PubMed] [Google Scholar]
- GRABAR P. The use of immunochemical methods in studies on proteins. Adv Protein Chem. 1958;13:1–33. doi: 10.1016/s0065-3233(08)60597-5. [DOI] [PubMed] [Google Scholar]
- LARGIER J. F. A purification and investigation of tetanus antitoxin. Arch Biochem Biophys. 1958 Oct;77(2):350–367. doi: 10.1016/0003-9861(58)90082-1. [DOI] [PubMed] [Google Scholar]
- ORLANS E. S., JONES V. E. beta-Propiolactone as a toxoiding agent. Nature. 1958 Nov 1;182(4644):1216–1217. doi: 10.1038/1821216a0. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER A. M., Jr, YONEDA M. A reinvestigation of the diphtheria toxin-antitoxin flocculation reaction using 355-methionine-labelled toxin. Br J Exp Pathol. 1957 Apr;38(2):194–206. [PMC free article] [PubMed] [Google Scholar]
- POPE C. G., STEVENS M. F. The purification of antitoxin by absorption of nonantitoxic antibodies. Br J Exp Pathol. 1953 Feb;34(1):56–72. [PMC free article] [PubMed] [Google Scholar]










