Abstract
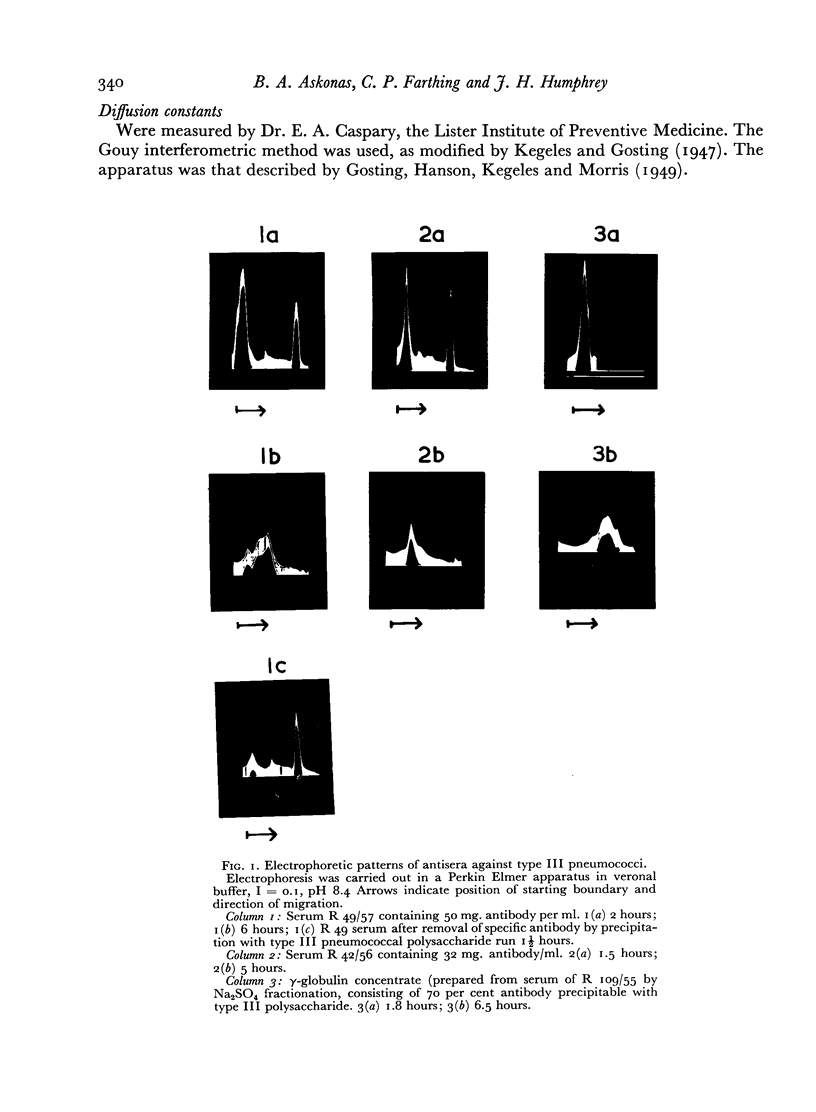
The electrophoretic patterns of six sera from rabbits immunized by two or more courses of intravenous injections of killed pneumococci type III showed multiple peaks in the γ-globulin region. Such sera contained large amounts of antibody (up to 85 per cent of the total γ globulin) against the capsular polysaccharide. One serum contained a cryoglobulin, which contained almost as great a proportion of specific antibody as did the remaining γ globulin.
The electrophoretic patterns and antibody contents were similar in the water-soluble and water-insoluble fractions of γ globulin.
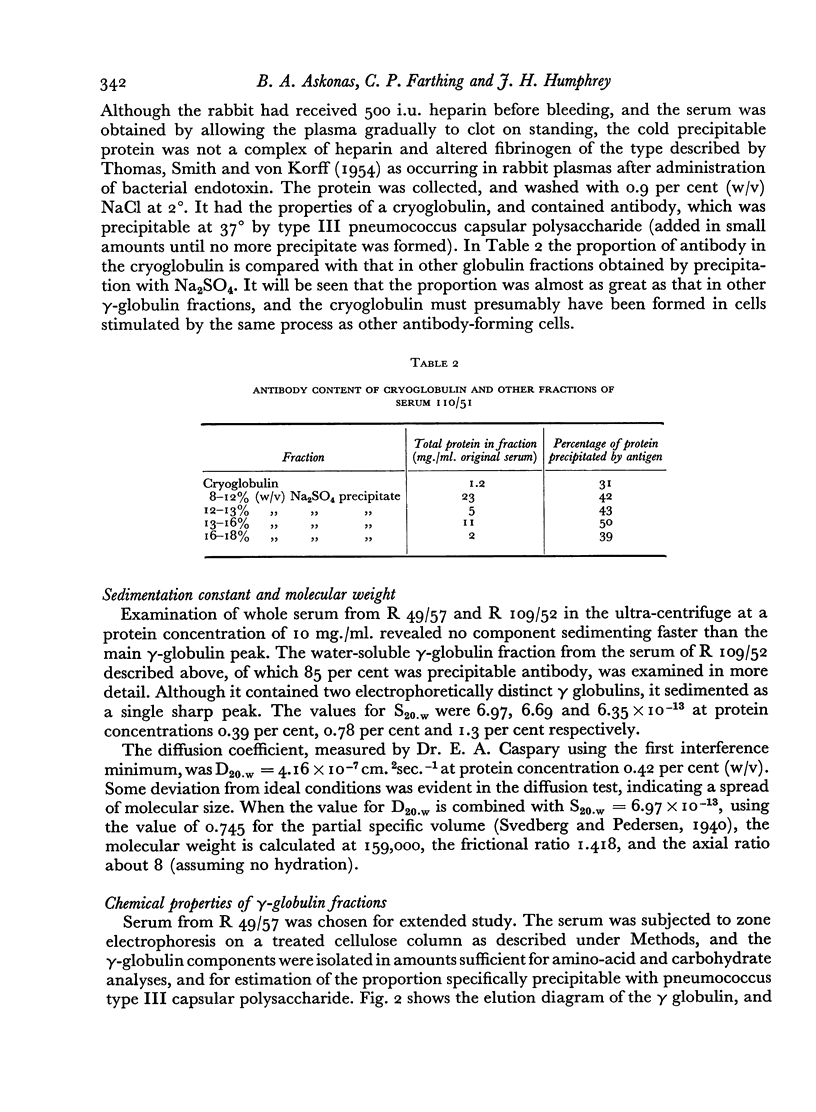
The sedimentation constant and diffusion coefficient of a water-soluble fraction of γ globulin, containing 85 per cent specific antibody, were measured. The values, at 0.4 per cent protein concentration, were S20.w = 6.97×10-13 and D20.w = 4.16×10-7 cm.2 sec.-1, corresponding to molecular weight 159,000.
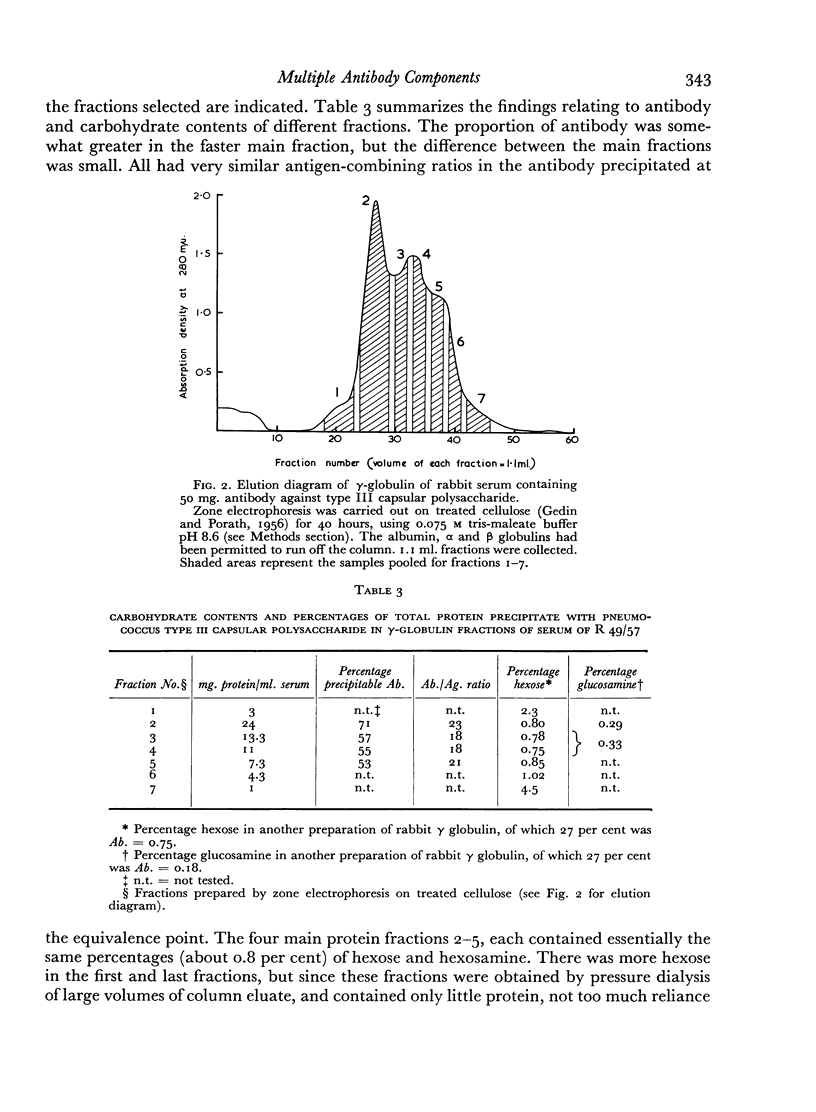
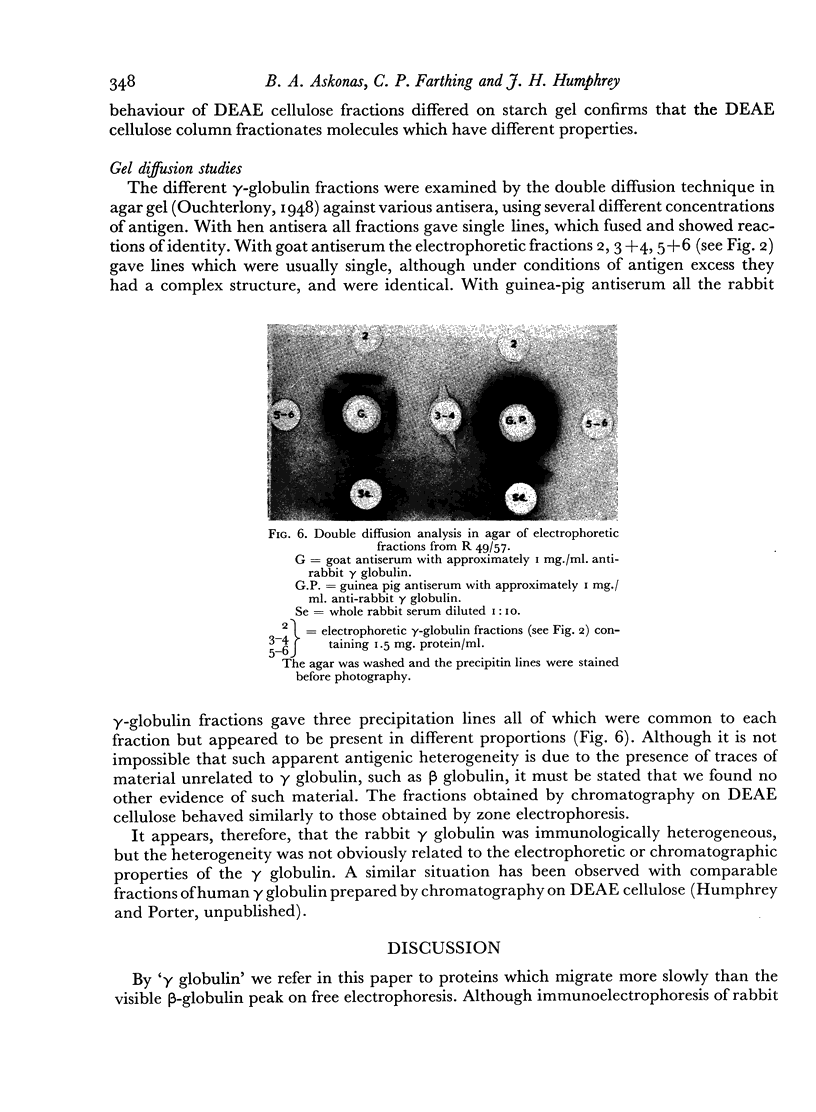
The antibody-containing globulin from one serum was separated by zone electrophoresis into three fractions with different electrophoretic mobilities. These contained 53–71 per cent of antibody precipitable by type III pneumococcus capsular polysaccharide. Only doubtfully significant differences were found in respect of amino-acid composition, hexose and hexosamine contents, or antigenic characteristics.
A method was devised for detecting small amounts of antibody against capsular polysaccharide by means of red cells sensitized with culture filtrates of capsulated pneumococci.
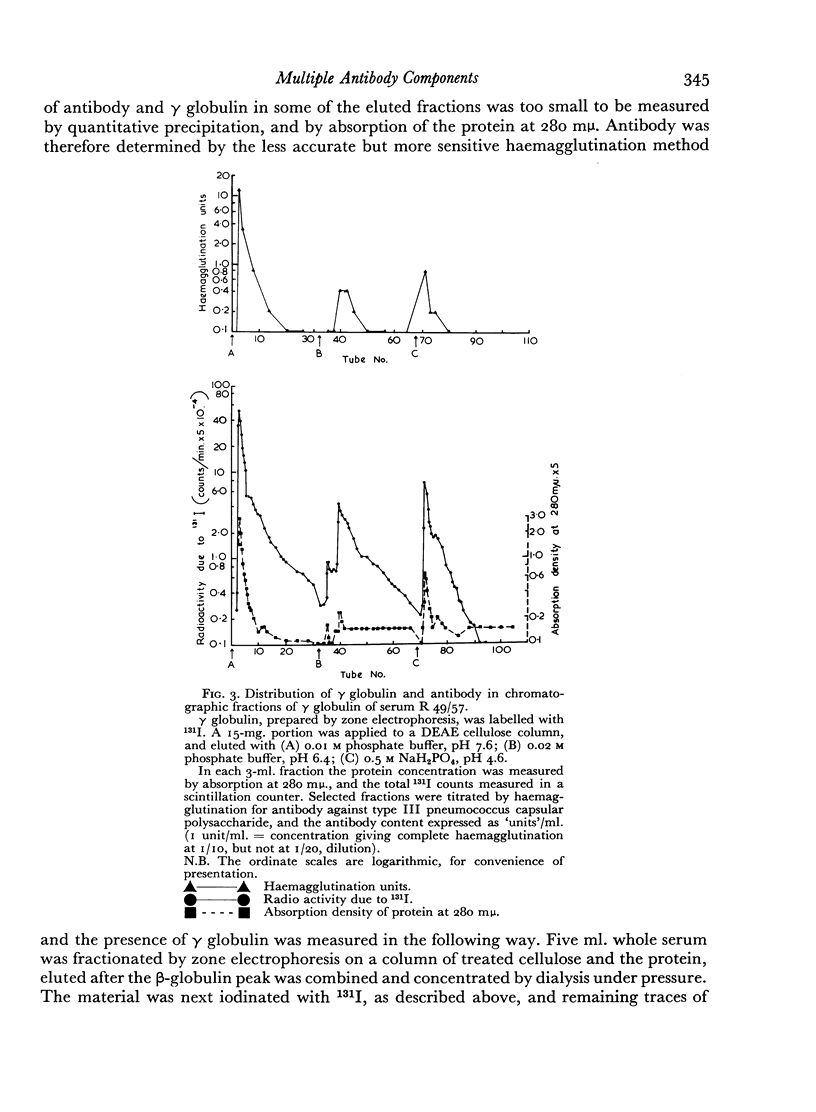
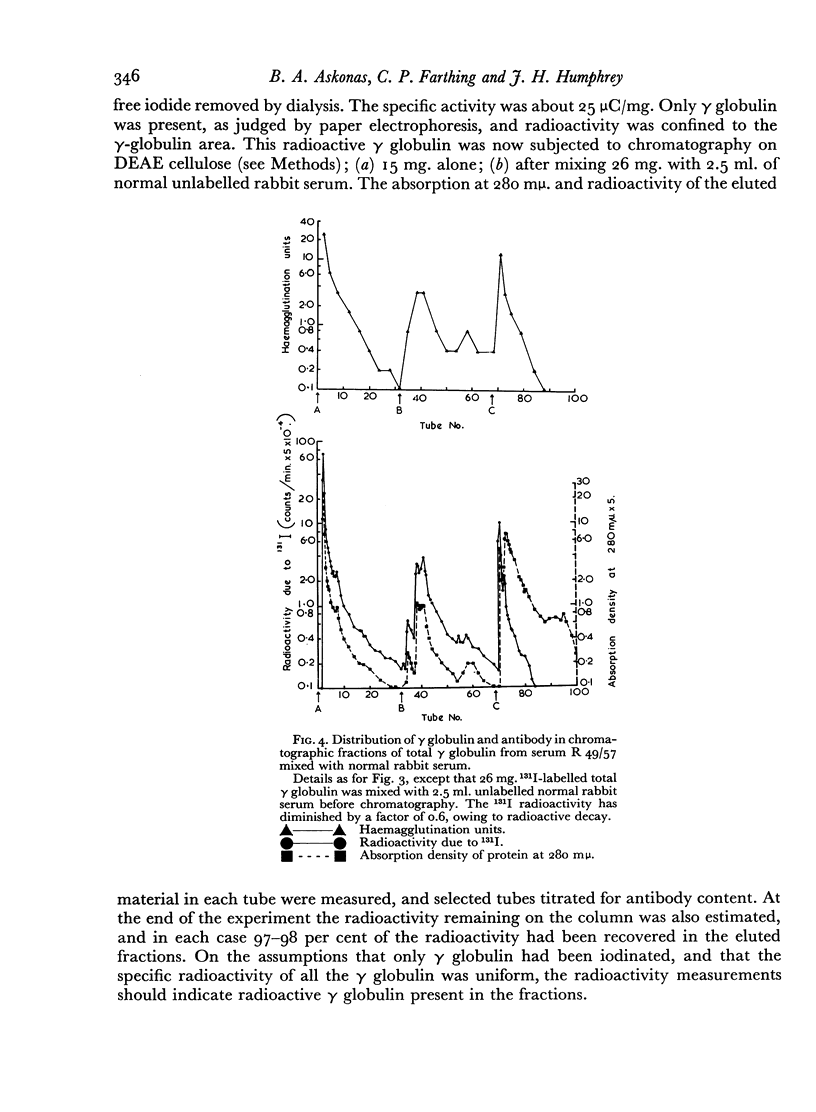
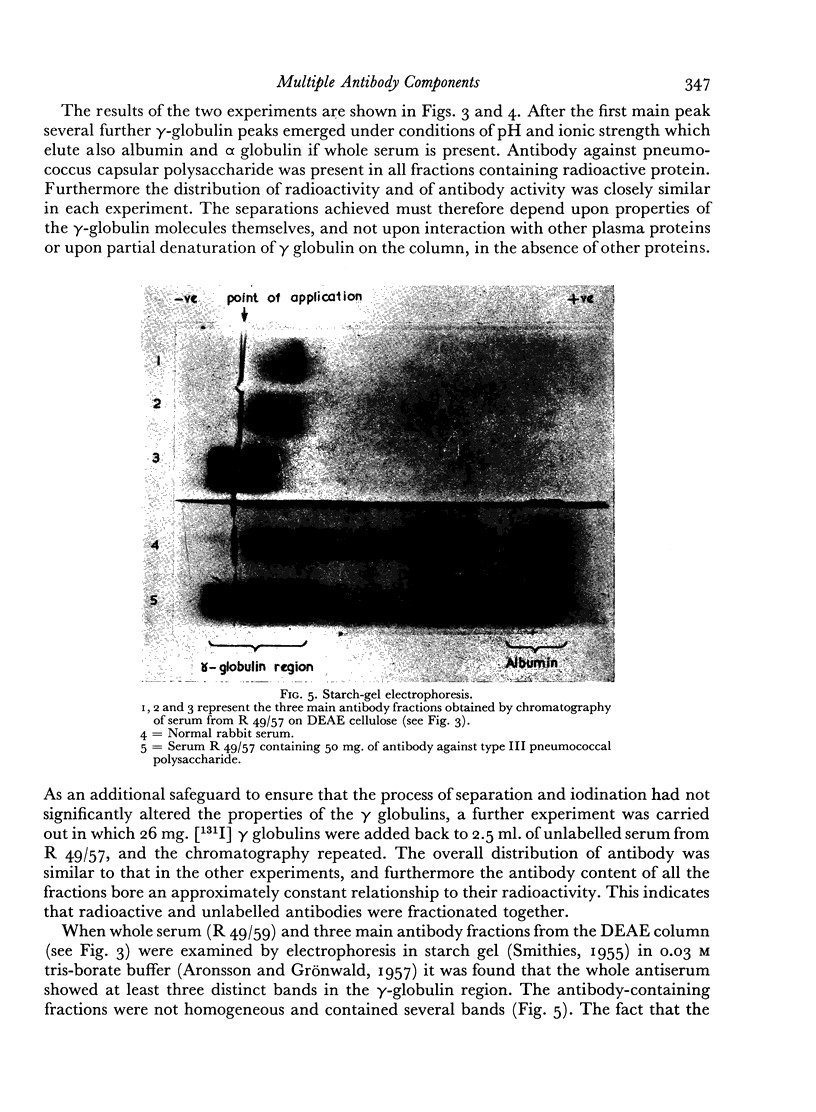
The antibody was also fractionated by chromatography on anion-exchange cellulose, and numerous fractions with antibody activity were obtained. It was shown by labelling the γ globulin with 131I that similar fractionation occurred both in the presence and absence of other serum components. All the chromatographic fractions of γ globulin were found to contain approximately similar proportions of antibody. By electrophoresis in starch gel the fractions were found to differ from one another and to be heterogeneous.
The implications are discussed of the finding that antibody against type III pneumococcus capsular polysaccharide can occur over the entire range of γ-globulin molecules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSSON T., GRONWALL A. Improved separation of serum proteins in paper electrophoresis: a new electrophoresis buffer. Scand J Clin Lab Invest. 1957;9(4):338–341. doi: 10.1080/00365515709079983. [DOI] [PubMed] [Google Scholar]
- ASKONAS B. A., HUMPHREY J. H., PORTER R. R. On the origin of the multiple forms of rabbit gamma-globulin. Biochem J. 1956 Jul;63(3):412–419. doi: 10.1042/bj0630412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- FAHEY J. L., HORBETT A. P. Human gamma globulin fractionation on anion exchange cellulose columns. J Biol Chem. 1959 Oct;234:2645–2651. [PubMed] [Google Scholar]
- GEDIN H. I., PORATH J. Studies of zone electrophoresis in vertical columns. II. Zone electrophoresis of serum proteins. Biochim Biophys Acta. 1957 Oct;26(1):159–169. doi: 10.1016/0006-3002(57)90067-7. [DOI] [PubMed] [Google Scholar]
- HAYES L. Specific serum agglutination of sheep erythrocytes sensitized with bacterial polysaccharides. Aust J Exp Biol Med Sci. 1951 Jan;29(1):51–62. doi: 10.1038/icb.1951.7. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOKRASCH L. C. Analysis of hexose phosphates and sugar mixtures with the anthrone reagent. J Biol Chem. 1954 May;208(1):55–59. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. The carbohydrate of gamma-globulin and myeloma proteins. J Exp Med. 1956 Aug 1;104(2):253–269. doi: 10.1084/jem.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., McFADDEN M. L., STOCKELL A., BUETTNER-JANUSCH V. Amino acid composition of four rabbit antibodies. J Biol Chem. 1955 May;214(1):197–207. [PubMed] [Google Scholar]
- THOMAS L., SMITH R. T., VON KORFF R. Cold-precipitation by heparin of a protein in rabbit and human plasma. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):813–818. doi: 10.3181/00379727-86-21241. [DOI] [PubMed] [Google Scholar]