Abstract
Fowl antibodies to rabbit γ globulin, bovine serum albumin and to ε toxin were studied by means of their reactions with rabbit antisera to fowl globulin. Specific precipitates redissolved either in antigen excess or in solutions of lower salt concentration than those in which they had been formed were used to measure the sedimentation and diffusion coefficients and the electrophoretic mobilities of the soluble complexes of fowl antibody with antigen.
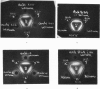
Fowl antiserum to any one antigen was found to contain two types of homologous antibodies, derived from different constituents of serum, having the same electrophoretic mobility but giving two distinct bands of precipitation with rabbit anti-fowl-globulin serum. The values obtained for the molecular weights of soluble complexes indicate that one type of antibody has a molecular weight of about 600,000 and the other 180,000, and also suggest that the latter type combined with only one molecule of antigen in antigen excess. Both types of antibody were precipitated by antigen in 0.9 and 8 per cent NaCl; the larger precipitates formed at the higher salt concentration contained more of the low molecular weight antibody, together with another component that was neither antigen nor antibody.
Full text
PDF


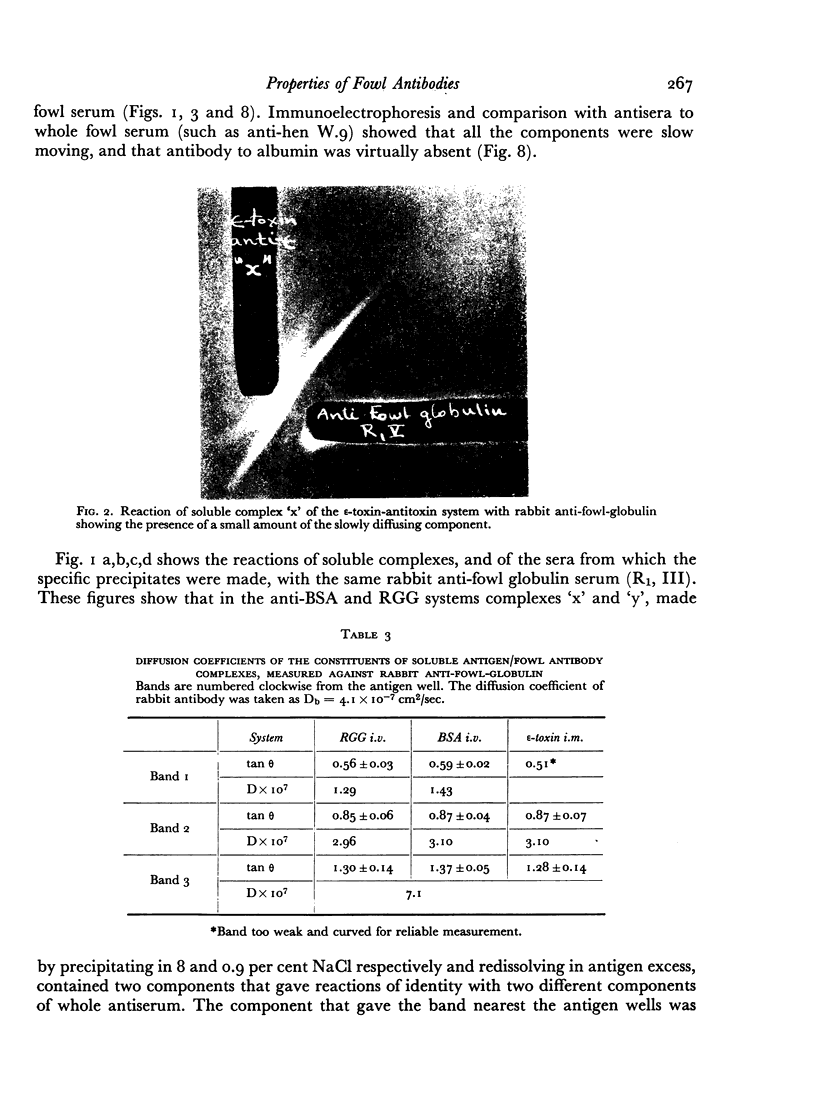
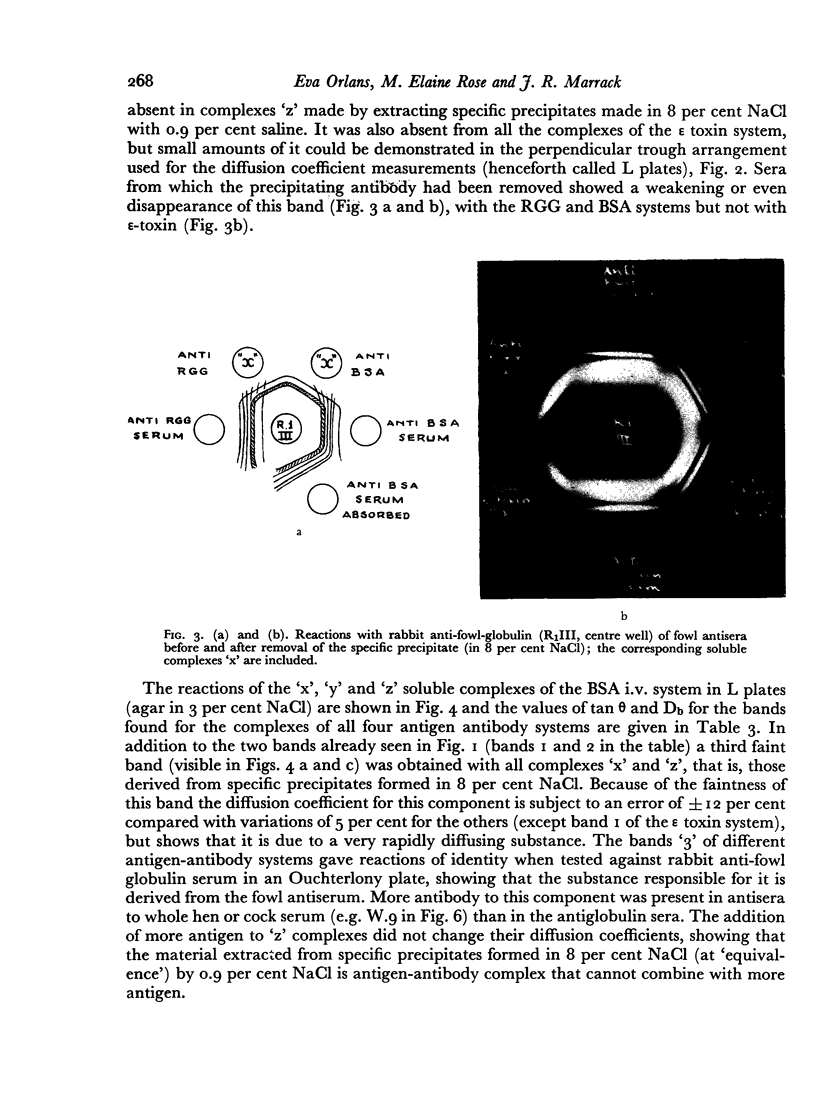
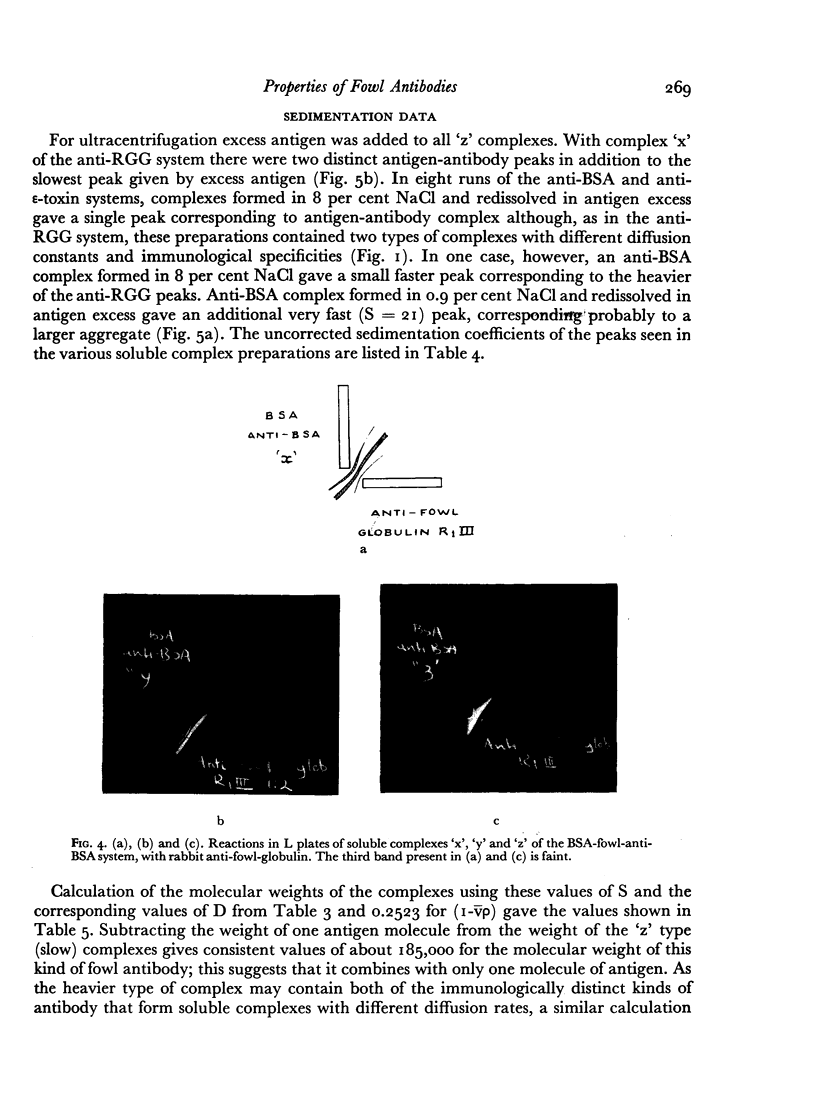
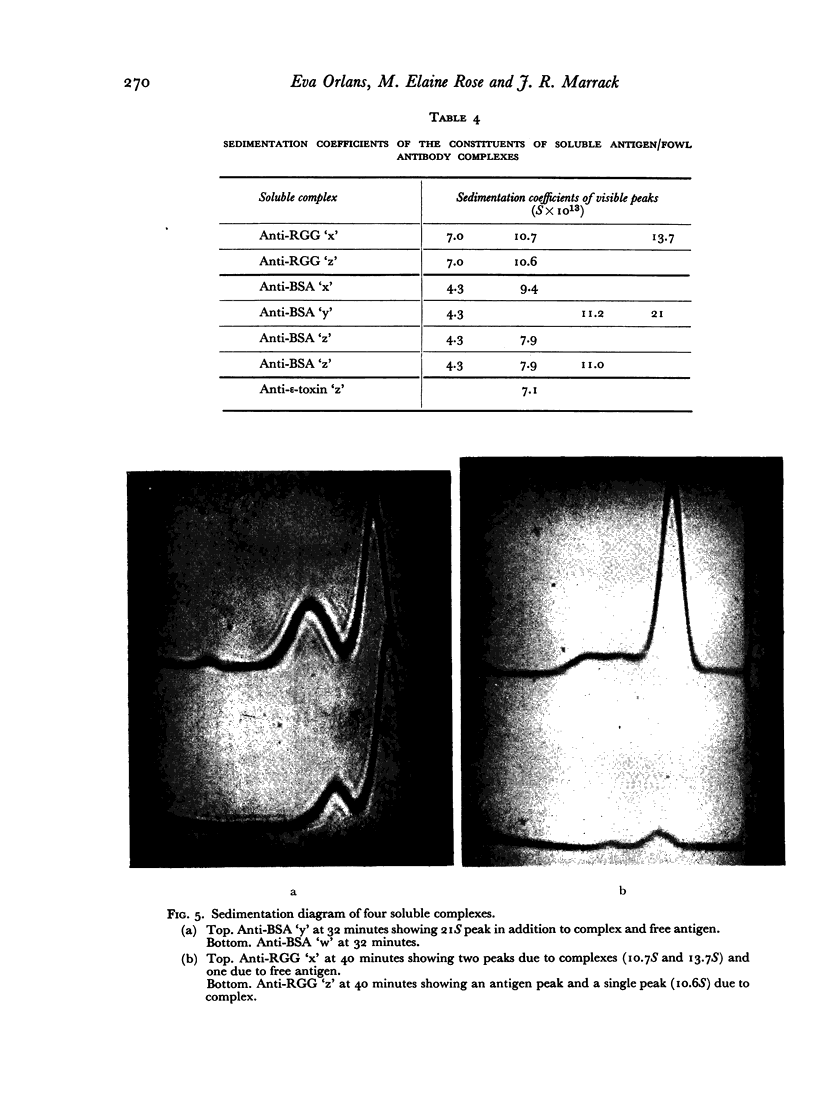
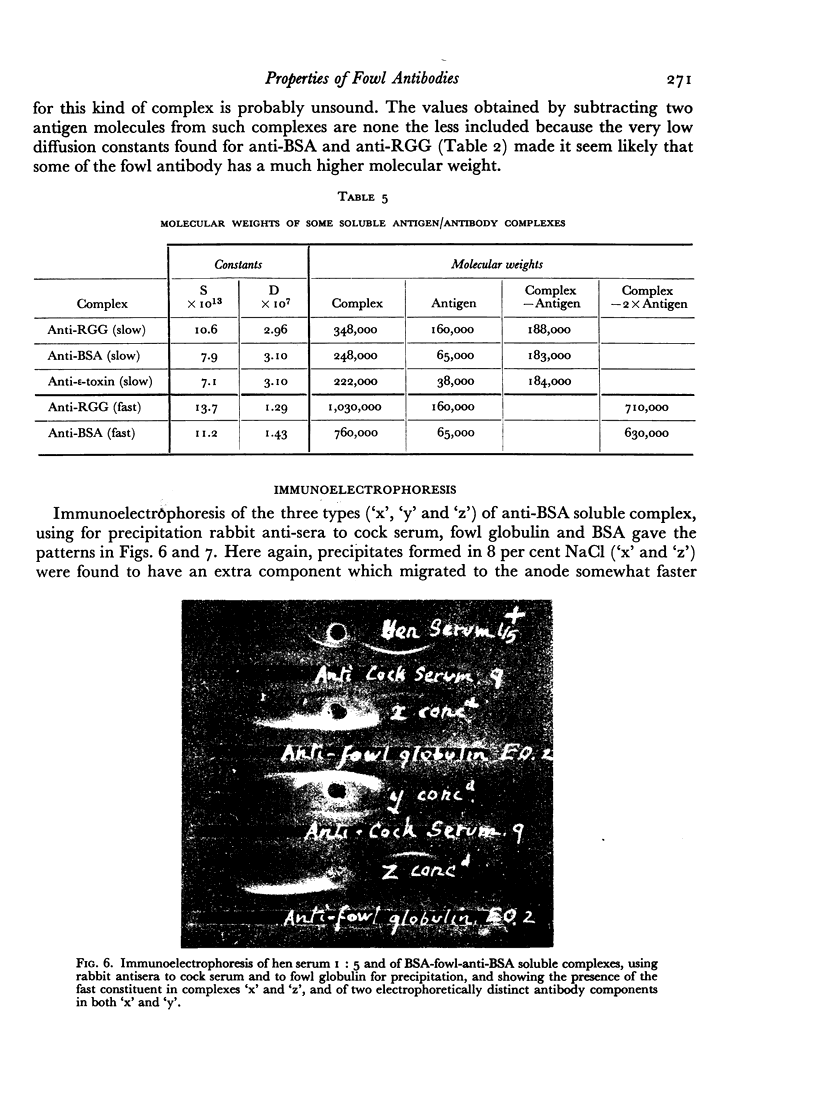

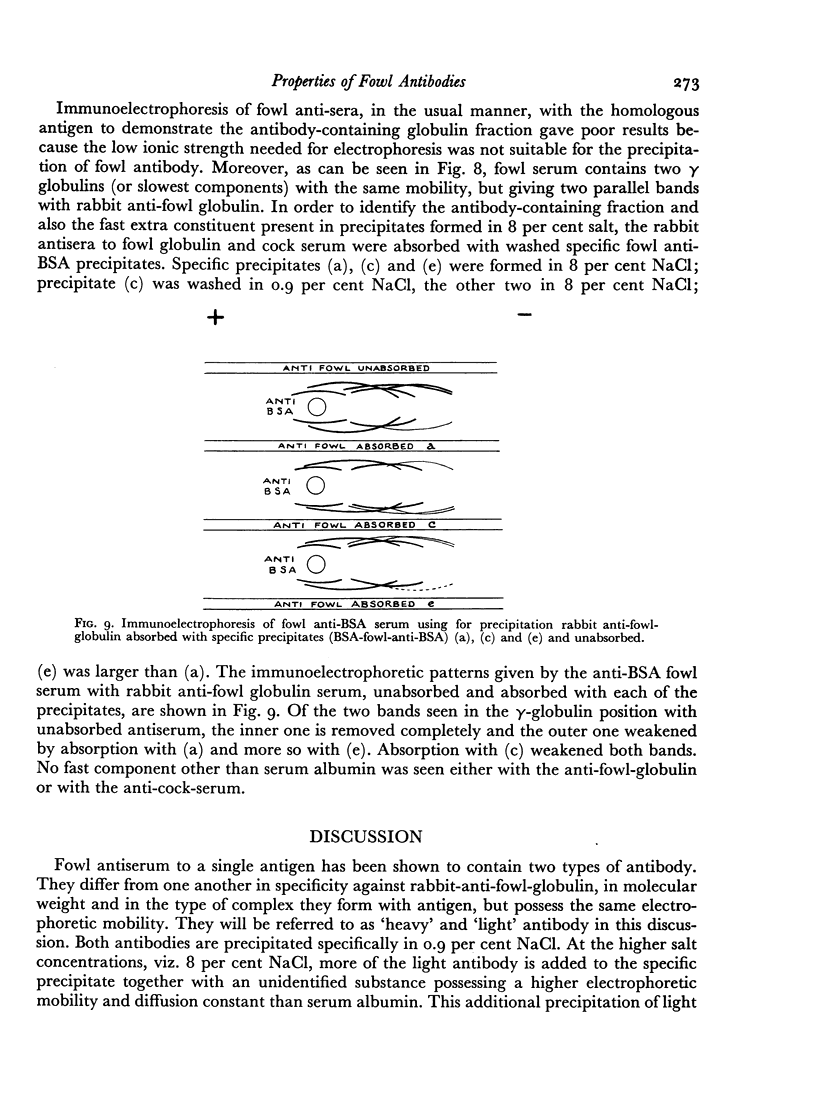




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANOVITZ J., SINGER S. J., WOLFE H. R. Precipitin production in chickens. XVIII. Physical chemical studies on complexes of bovine serum albumin and its chicken antibodies. J Immunol. 1959 Jun;82(6):481–488. [PubMed] [Google Scholar]
- BUXTON A. On the transference of bacterial antibodies from the hen to the chick. J Gen Microbiol. 1952 Nov;7(3-4):268–286. doi: 10.1099/00221287-7-3-4-268. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., NICHOL J. C., COHN M. Biophysical studies of blood plasma proteins; immunological and electrophoretic studies of immune chicken serum. J Immunol. 1949 Oct;63(2):195–210. [PubMed] [Google Scholar]
- GOODMAN M., WOLFE H. R., NORTON S. Precipitin production in chickens. VI. The effect of varying concentrations of NaCl on precipitate formation. J Immunol. 1951 Feb;66(2):225–236. [PubMed] [Google Scholar]
- MAKINODAN T., GENGOZIAN N., CANNING R. E. Demonstration of a normal serum macroglobulin coprecipitating with the bovine serum albumin (BSA)-chicken anti-BSA precipitate. J Immunol. 1960 Nov;85:439–446. [PubMed] [Google Scholar]
- MARRACK J. R., HOCH H., JOHNS R. G. S. The valency of antibodies. Br J Exp Pathol. 1951 Jun;32(3):212–230. [PMC free article] [PubMed] [Google Scholar]
- ORLANS E. S., RICHARDS C. B., JONES V. E. Clostridium welchii epsilon-toxin and antitoxin. Immunology. 1960 Jan;3:28–44. [PMC free article] [PubMed] [Google Scholar]
- RAYNAUD M., RELYVELD E. H. [Diphtheria toxin-antitoxin reaction]. Ann Inst Pasteur (Paris) 1959 Nov;97:636–678. [PubMed] [Google Scholar]
- WOLFE H. R., MUELLER A. P., NEESS J. C. Physical factors affecting maximum precipitation of the BSA-AntiBSA fowl system. Immunology. 1959 Jul;2:195–202. [PMC free article] [PubMed] [Google Scholar]