Abstract
We provide both molecular and pharmacological evidence that the metabotropic, purinergic, P2Y6, P2Y12 and P2Y13 receptors and the ionotropic P2X4 receptor contribute strongly to the rapid calcium response caused by ATP and its analogues in mouse microglia. Real-time PCR demonstrates that the most prevalent P2 receptor in microglia is P2Y6 followed, in order, by P2X4, P2Y12, and P2X7 = P2Y13. Only very small quantities of mRNA for P2Y1, P2Y2, P2Y4, P2Y14, P2X3 and P2X5 were found. Dose-response curves of the rapid calcium response gave a potency order of: 2MeSADP>ADP=UDP=IDP=UTP>ATP>BzATP, whereas A2P4 had little effect. Pertussis toxin partially blocked responses to 2MeSADP, ADP and UDP. The P2X4 antagonist suramin, but not PPADS, significantly blocked responses to ATP. These data indicate that P2Y6, P2Y12, P2Y13 and P2X receptors mediate much of the rapid calcium responses and shape changes in microglia to low concentrations of ATP, presumably at least partly because ATP is rapidly hydrolyzed to ADP. Expression of P2Y6, P2Y12 and P2Y13 receptors appears to be largely glial in the brain, so that peripheral immune cells and CNS microglia share these receptors. Thus, purinergic, metabotropic, P2Y6, P2Y12, P2Y13 and P2X4 receptors might share a role in the activation and recruitment of microglia in the brain and spinal cord by widely varying stimuli that cause the release of ATP, including infection, injury and degeneration in the CNS, and peripheral tissue injury and inflammation which is signaled via nerve signaling to the spinal cord.
Keywords: mouse, ATP receptors, glial cells, purines, pain, injury
INTRODUCTION
Investigators have hypothesized recently that microglia, the major intrinsic immune competent cells of the brain and spinal cord, play a major role in enhancing the long-term pain that accompanies nerve injury and peripheral tissue injury and inflammation (Colburn et al., 1999; Arruda et al., 2000; Fu et al., 2000; Watkins et al., 2001a; Watkins et al., 2001b; Svensson et al., 2003; Tsuda et al., 2003; Raghavendra et al., 2004). However, the mechanisms by which peripheral injury and inflammation recruit and activate the normally ‘resting’ microglia (Streit et al., 1988) and the mechanisms by which activated microglia enhance pain are only poorly understood. Our laboratory (Wu et al., 2004) recently determined that P2 receptors are likely mediators for both the activation of microglia and long-term pain caused by peripheral injury. Tsuda et al. 2003 suggest that activating P2 receptors on microglia is also at least partly responsible for neuropathic pain. To further understand which P2 receptors on microglia are responsible for the activation of microglia we conducted the present molecular and functional experiments.
Although many cytokines including fractalkine can activate microglia (reviewed in Hanisch, 2002), another substance that is released in relatively high concentrations by all tissues following all types of injury is adenosine triphosphate (ATP). In addition, ATP is released by primary afferents and by astrocytes under several circumstances (Salter et al., 1993; Hassinger, et al., 1996; Bardoni et al., 1997; Guthrie, et al., 1999). It has been suggested that ATP and its metabolites might be used for communicating between different glia types and neurons (Hansson and Ronnback, 2003). Thus, it might be that injury and inflammation utilize ATP to coordinate the activation of neurons, astrocytes and microglia to centrally enhance pain signaling.
Tsuda et al. 2003 and Inoue et al. 2004 suggest that ATP acting via the P2X4 purinergic receptor might have a major role in activating microglia and causing enhanced pain following spinal nerve injury. However, other labs have suggested that functional metabotropic P2Y receptors including P2Y12 receptors mediate the chemotaxis that occurs during activation (Moller et al., 2000; Honda et al., 2001). Our laboratory has also suggested that P2Y receptors might play a role in activation of microglia and long-term pain following formalin injection into the paw in rodents (Wu et al., 2004). A recent report (Bianco et al., 2005) suggests that all P2X and P2Y receptors are expressed by microglia, based on rtPCR. However, this group relied on microglia cell lines for most of the PCR data.
In this report, we have used rtPCR and real-time quantitative PCR to demonstrate that P2Y6, P2Y12, P2Y13 and P2X4 receptors are expressed in mouse microglia in culture, and in the mouse spinal cord and brain. Real-time quantitative PCR demonstrates that some P2Y and P2X receptors have low expression in microglia, whereas those listed above are abundantly expressed. We also used agonists and antagonists that activate rapid increases in intracellular calcium and rapid shape changes in microglia to conclude that either ATP or its metabolite ADP activates purinergic receptors with properties that are most like the newly cloned P2Y12, and P2Y13 receptors in addition to P2Y6 and P2X4 receptors. We also confirm that activation of the P2X7 receptor might occur at higher, but physiological, doses of ATP and can lead to more sustained increases in intracellular calcium (Ferrari et al., 1996; Ferrari et al., 1997; Verderio and Matteoli, 2001).
OBJECTIVE
To determine the molecular P2 receptor subtypes in mouse microglia in culture and the functional expression of the P2 receptor subtypes using selective agonists and antagonists, and to use imaging of intracellular calcium increases and shape changes as indicators of P2 receptor activation.
METHODS
Culture methods
All guidelines required by University of Utah’s and University of North Carolina’s IACUCs were followed in harvesting tissues. Glia were obtained from P0–P7 C57BL/6 mouse pups using the methods described previously (Guthrie et al., 1999). Briefly, cortices were removed, cleaned of meninges and trypsinized, and triturated to dissociate. Cells were plated in culture flasks at 50 000 cells cm2, and grown in modified eagles medium (MEM), 10% fetal calf serum, penicillin and streptomycin, essential amino acids and nonessential amino acids. Microglia were harvested from astrocytes that were confluent and not more than 3-weeks old. Passaging microglia was accomplished by shaking and slapping the flask on a table, several times and vigorously swirling the flasks to dislodge microglia attached to the monolayer of astrocytes. The growth medium containing the dislodged microglia cells was centrifuged at 800 rpm for 5 minutes. Following centrifugation, most of the supernatant was removed and the cells in the pellet were resuspended in the remaining 2–3 ml, yielding a density of ~85 000 cells ml−1. The density of cells was ~20 000 cells cm2 after plating.
Cells were passaged into wells of a Falcon 24- well Multiwell plate (35-3047). Each well was pretreated with 266 μg ml−1 poly-L-lysine then dried. Silicone gaskets with an inner diameter of 4 mm were used to reduce the plating surface in each well. 20–30 μl of microglia suspension was placed into the center of each gasket of 12 wells in each multiwell plate. Multiwell plates were placed in the incubator for 1 hour, then 1 ml MEM + 10% FBS was added. All above procedures were conducted at 37°C, with 5% CO2 atmosphere. Microglia purity was assessed using GSA-IB4 lectin, and OX-42-antibody- (Serotec) labeling, which labels the CR3 receptor. These indicated >90% pure microglia with astrocytes, and oligodendroglia as the most common contaminants. Ca2+ responses from non-microglia were not included in the data analysis. Observations were made 4 hours and 24 hours after passaging.
RNA expression
Cultures
Microglia from mouse brain were prepared and partially isolated as described above (>90% microglia/ <10% astrocytes and oligodendrocytes). In addition, the culture bed (astrocytes, microglia, oligodendrocytes and a few neurons) was also detached from the culture flasks with trypsin and pelleted. Total RNA was isolated from the cells using the RNAeasy extraction kit from Qiagen and rtPCR was performed on 1 μg of total RNA, for 37 cycles: each cycle was 95°C for 14 seconds, 57°C for 30 seconds, then 68°C for 1 minute. PCR primers for P2Y receptors were designed from the genomic sequences obtained from the NCBI GenBank sequence database. Table 1 lists the characteristics of these primers. Analysis of 5 μl of the PCR product was by electrophoresis on agarose gels with ethidium bromide.
Table 1.
PCR primers
| P2Y | Accession number | 5′ primer | 3′ primer | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| P2Y1 | gi6679192 | 239–260 | 946–925 | 708 | 56.6 |
| P2Y2 | gi6679194 | 331–353 | 920–898 | 590 | 59.8 |
| P2Y4 | gi10181171 | 606–627 | 1055–1034 | 450 | 58.7 |
| P2Y6 | gi10945622 | 69–90 | 907–883 | 839 | 60.3 |
| P2Y12-1 | gi21426814 | 88–110 | 602–579 | 515 | 55.8 |
| P2Y12-2 | gi21426814 | 54–73 | 992–973 | 939 | 54.3 |
| P2Y13-1 | gi27229196 | 39–62 | 291–269 | 253 | 57.5 |
| P2Y13-2 | gi27229196 | 492–513 | 1669–1646 | 1178 | 55.7 |
Brain
Total RNA was extracted from brain and spinal cord tissue following perfusion with 0.1 M phosphate buffered saline using TRIZOL reagent (Invitrogen). We performed rtPCR and analyzed as described for the cell-culture RNA above. Primers coding for larger products for P2Y12 and 13 were used for the brain extracts to ensure specificity. Product from these gels was sequenced and returned results >95% identical to the known sequences for PY12 and 13.
Real-time PCR
In addition to microglia, harvested as described above, ‘mixed glia’ (mostly astrocytes and microglia) and ‘purified microglia’ (>90% microglia by visual inspection) were prepared. All harvest types were reduced to a pellet plus a minimum amount of medium (10–30 μl), 5 μl RNAse-out (Invitrogen 10777-019) and 50 μl RNA-Later (Qiagen 1017980) was added and mixed quickly before quick freezing in a dry ice/methanol slurry. Mixed glia were harvested from monolayers after first shaking the flask to remove loose and suspended microglia. Flasks were then quickly rinsed with 5 ml 0.05% Trypsin/EDTA Invitrogen 25300-054), and incubated in 5 ml fresh 0.05% Trypsin/EDTA. Cells loosened by Trypsin treatment were centrifuged and quick frozen as described above. To harvest ‘purified microglia’, four microglia pellets from 75 cm2 flasks were passaged into one 25 cm2 flask in 3 ml MEM and FBS, and incubated for 4 hours at 37°C. Following incubation the cells loosened by shaking were centrifuged and quick frozen as described above.
The brain and spinal cord were also removed from adult mice euthanized with an overdose of Halothane, and the cerebral cortex and spinal cord quickly removed and frozen in liquid nitrogen. Following thaw in RNeasy disruption buffer, samples were homogenized using pipettes. For both preparations, the protocol below was followed.
RNA was extracted from frozen pellets using the RNeasy Mini Kit (Qiagen 74104). Immediately following RNA extraction and purification, extracts were converted to cDNA using the SuperScript III First-Strand Synthesis System for rtPCR (Invitrogen 18080-051). DNA concentrations were determined using OD measurements. The cDNA libraries were analyzed using the ABI quantitative real-time PCR system on the ABI Prism 7900 Sequence Detection System (Advanced Biosystems Inc.). ABI supplied ‘Taqman Gene Expression Assay’ primer and probe sets (both standard and custom) for P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, P2Y13, P2Y14, P2X3, P2X4, P2X5, P2X7, glial filament acidic protein (GFAP), β-Actin (Act b), toll-like receptor 4 (tlr-4) and CD14 (see Table 2 for specific primers) were used with ABI Taq-Man Master mix. Template concentrations were normalized prior to addition to reaction wells in a 96-well reaction plate. Each sample was run in triplicate with one ‘no-template’ control. GFAP was used to assess the degree of astrocyte presence in the purified microglia samples from which total RNA was extracted. In addition, Mac-1 (CR3 receptor), (tlr-4) and CD14 mRNA expression were assessed as markers for microglia (CD14 antigen is part of the functional heteromeric lipopolysaccharide (LPS) receptor complex comprised of CD14, tlr4 and MD-2) (Underhill and Ozinsky, 2002).
Table 2.
Taqman Gene-Expression Assay primer and probe sets for real-time PCR analyses (supplied by ABI)
| Product number | Abbreviation | Product |
|---|---|---|
| Mm00607939_s1 | Actb | β-Actin |
| Mm00546086_m1 | Gfap | GFAP |
| Mm00438094_g1 | CD14 | CD14 |
| Mm00434455_m1 | Itgam | Mac-1 CR3 receptor |
| Mm00445274_m1 | Tlr4 | Tlr-4 |
| Mm00435471_m1 | P2ry1 | P2Y1 receptor |
| Mm00435472_m1 | P2ry2 | P2Y2 receptor |
| Mm00445136_s1 | P2ry4 | P2Y4 receptor |
| 4331348 p2y6 custom | p2y6 | P2Y6 receptor |
| Mm00446026_m1 | P2ry12 | P2Y12 receptor |
| Mm00546978_m1 | P2ry13 | P2Y13 receptor |
| Mm01283360_m1 | P2Y14 | P2Y14 receptor |
| Mm00523699_m1 | P2X3 | P2X3 receptor |
| Mm00501787_m1 | P2rx4 | P2X4 receptor |
| Mm00473677_m1 | P2X5 | P2X5 receptor |
| Mm00440582_m1 | P2X7 | P2X7 receptor |
The relative amount of astrocyte and microglia markers, and P2Y and P2X receptor RNA was computed using the Standard Curve Methods and Comparative Ct Methods described in Applied Biosystems User Bulletin #2.
Statistical analysis consisted of one way analysis of variance followed by comparisons to control using Dunnet’s test.
Calcium imaging
All experiments were performed on a calcium-imaging setup consisting of a Nikon DiaPhot 300 microscope with custom-built epifluorescence illumination. Illumination wavelength and intensity were controlled using a computer-controlled filter wheel. Images were acquired with a Photometrics cooled CCD camera employing a Kodak KAF-1400 chip (Roper Scientific). The microscope, camera and manipulators were mounted on a vibration isolation table (Newport Corp.).
Cells were loaded for 30–50 minutes in growth medium containing the calcium indicator fluo-3 AM (3 μM; Molecular Probes), washed two times with observation medium, then allowed 20–30 minutes to de-esterify and to come to room temperature. Other experiments used fura-4, loaded similar to the description above, as the calcium indicator. These experiments provided more quantitative data on intracellular calcium concentrations. Sequences of images were acquired at 1.5-second intervals. Control intensities were based on 7–15 images collected before drug administration. The control images were also used to identify the positions of cells according to minimum intensity and minimum size criteria; high-pass filtered images were used to help define the borders of the cells. The average intensity of each cell (usually 100–200 cells field−1) was measured in each image of the sequence. The criterion for a positive response was set at a fluorescence intensity exceeding the control values by at least 2 standard deviations of those control values for at least two consecutive frames.
Drug administration
Drugs were administered via a custom chamber lowered into each well of the multiwell plate, which prevented changes in the liquid level within the well. The flow of drug solution was controlled with a peristaltic pump that delivered enough solution through the well to completely replace the well volume in 15 seconds. The well was perfused throughout the entire observation period. The perfusion system was rinsed thoroughly between applications. All calcium-imaging experiments were performed in observation medium (MEM with 10 mM Hepes and 25 mM glucose, pH 7.3) at room temperature.
Agonists used: UTP, UDP, ATP, ADP, 2-methylthio-ADP (2MeSADP), 2′,3′-O-(4-benzoyl-benzoyl) adenosine 5′-triphosphate (BzATP), adenosine 5′-O-(2-thiophosphate) (ADPβS) P1,P4-di-adenosine-5′ tetraphosphate (A2P4). Antagonists used: oxidized ATP (o-ATP), 2′-deoxy-N6-methyladenosine-3′,5′-bisphosphate (MRS 2179), P1,P4-di-adenosine-5′ tetraphosphate (A2P4). All drugs were diluted in observation medium (see above). 2MeSADP and pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) were obtained from RBI. All other nucleotides and suramin were obtained from Sigma. All nucleotides were purchased at the highest purity available. Pertussis toxin (PTx) was obtained from BIOMOL Laboratories (Plymouth Meeting).
Shape changes
Phase-contrast micrographs of the region analyzed for rapid calcium increases (comprising 50–300 cells) were taken before and after administration of agonists. These photomicrographs were analyzed in Photoshop, overlaying the before photo with the after photo, with variable opacity applied. Changes in shape and movement (changes in location) were detected easily in these overlays. Changes in shape were graded on a five-point scale by an observer blinded to the treatments in which 0 = no change, 1 = changes in shape in <10% of the cells, 2 = changes in 10–20% of the cells, 3 = changes in 20–40% of the cells, 4 = changes in >40% of the cells, and 5 = changes in all cells in the field.
Statistical comparisons were made using one way analysis of variance, and individual comparisons made using Student’s t-test with significance accepted at P<0.05.
RESULTS
Microglia plated for 4 hours had few processes and resembled activated microglia described by other groups (Fig. 1, right). By 24 hours most microglia had many processes and resembled the more ‘resting-like’ morphology noted by Möller et al. 2000 when microglia were grown in activated astrocyte medium (Fig. 1, left). In preliminary experiments, we kept careful track of the types of microglia (e.g. highly processed, round, highly phase-bright, heavy labeling with IB4 lectin or complement receptor antibodies OX-42, Mac-1) that demonstrated calcium responses to ATP, and found no correlation between the shape and the responses at any dose of ATP. We also cultured microglia in different concentrations of glycine and serine to determine if these amino acids, which are purported to adjust the activation state of microglia, had an effect on the ATP induced calcium response, and did not observe a major effect. We did, however, observe that cultures passaged for >4 days were less responsive than cultures observed at either 4 hours or 24 hours.
Fig. 1. Phase-contrast photomicrographs of microglia.
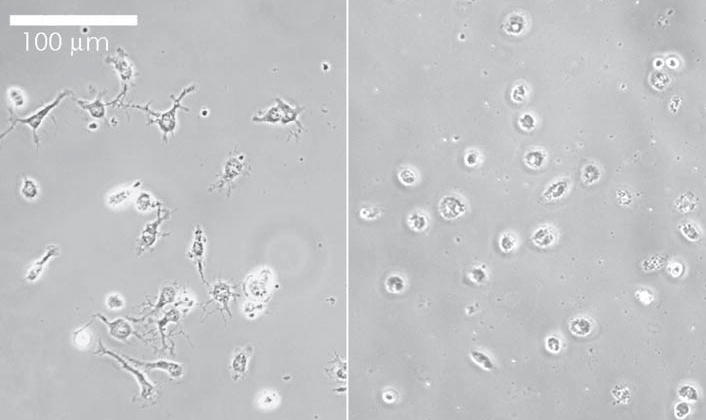
Both round, highly phase-bright microglia and multiprocessed microglia are visible. After plating for 4 hours (right), fewer microglia exhibit long branching processes than after 24 hours (left).
Receptor mRNA expression
Using rtPCR, we demonstrated that microglia cultures from mouse express RNA that encodes P2Y6, P2Y12 P2Y13 and P2X4 receptors (Fig. 2). Because the microglia cultures contain small numbers of astrocytes and oligodendroglia (<10% contamination), we compared the expression of P2Y receptors with the astrocyte cultures from which the microglia were harvested. Fig. 2 indicates that these mixed glial cultures express P2Y1, P2Y2, P2Y6, P2Y12 and P2Y13 and P2X4 receptor RNA. We also performed rtPCR on RNA extracts from whole mouse brain, and demonstrated the expression of both P2Y12 and P2Y13 receptor RNA (Fig. 3). However, rtPCR gave variable results, making it difficult to determine which of these receptors were expressed by microglia and which by astrocytes.
Fig. 2. rtPCR gel of P2 receptors from astrocytes and microglia in culture.
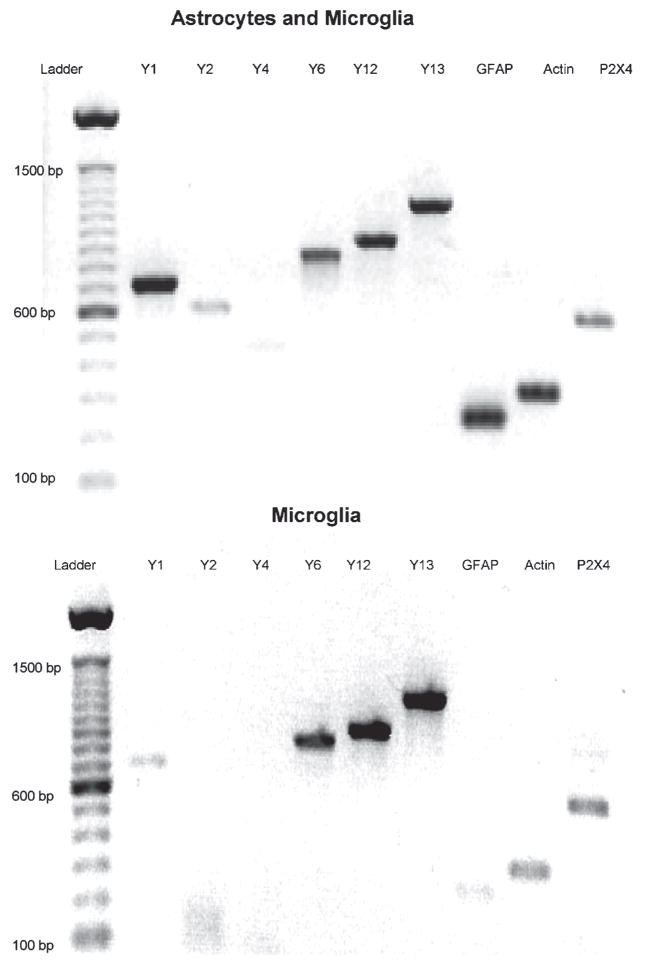
Note the presence of P2Y1 and P2Y2 receptors and GFAP in the mixed, astrocyte and microglia culture (top), and the large reduction of these in purified microglia (bottom). Predicted sizes of products for each lane are in Table 1.
Fig. 3. mRNA encoding P2Y12 and P2Y13 receptors in mouse brain.
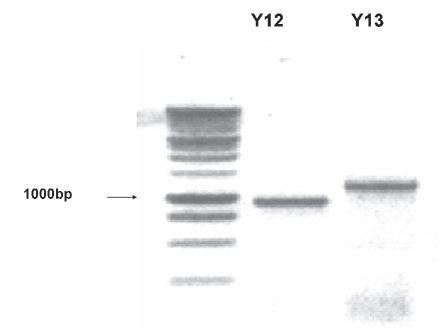
Gel of PCR products from adult mouse brain. The predicted sizes of the products for P2Y12 and P2Y13 are 939 bp and 1178 bp, respectively. The sequence of these products agreed >95% with published sequences.
Real-time quantitative PCR
Using semi-quantitative rtPCR, we noted the presence of GFAP and P2Y1 mRNA in the microglia preparations, and the bands of the various P2Y receptors varied from run to run. To get a better idea of the relative amounts of each of these receptor mRNAs, we used real-time PCR. As shown in Fig. 4, the expression of P2Y6, P2Y12, P2Y13 and P2X4 purinergic receptors in purified microglia is much higher than in the mixed glial cultures from which they were isolated. The mixed glial cultures from which the microglia were harvested had much greater expression of GFAP mRNA and less of the above receptors and the microglia markers Mac1 and CD14 (but not tlr-4) (Fig. 4). The mixed cultures also expressed more P2X7, which indicates that at least some of the P2X7 receptor is expressed by astrocytes.
Fig. 4. Real-time PCR of P2 receptor mRNA.
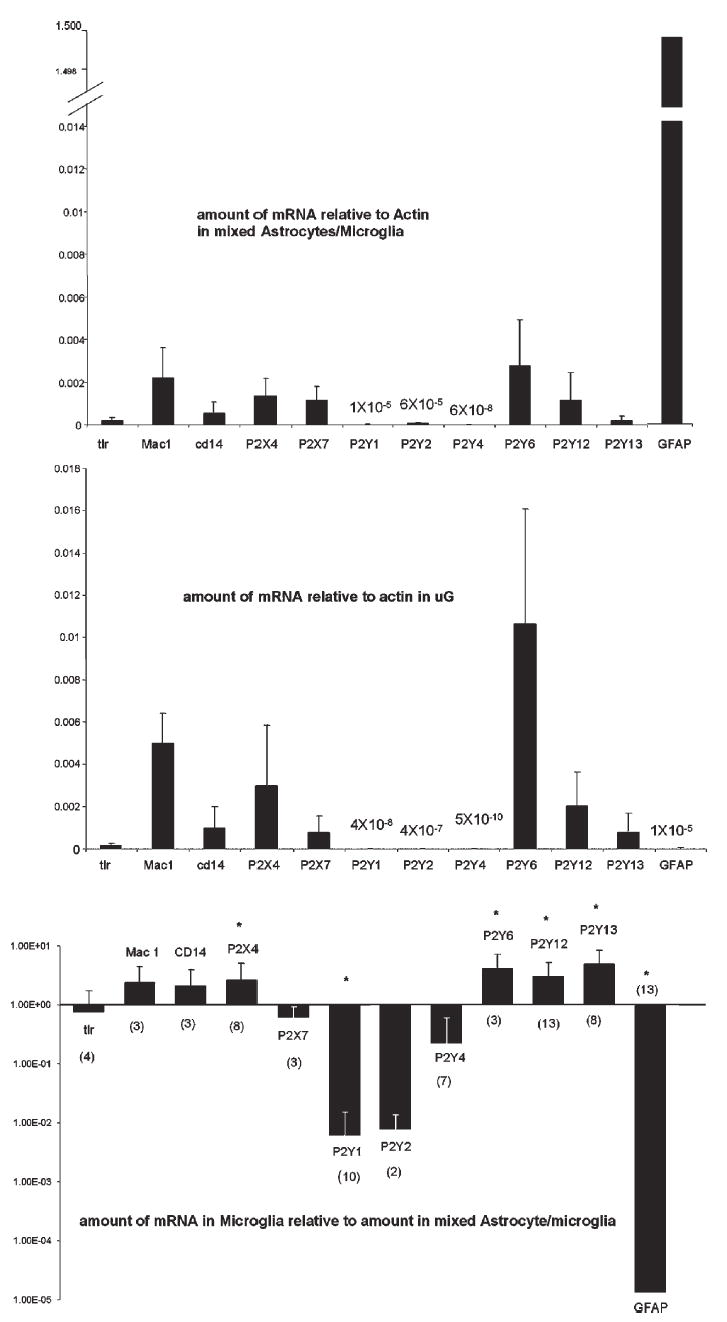
Top: Amount of mRNA of indicated markers and receptors relative to actin from purified microglia. Data mean ± S.D. Middle: Amount of mRNA relative to actin from mixed astrocyte/microglia cultures from which purified microglia were harvested. Note break in scale to include GFAP. Data mean ± S.D. Bottom: Amounts of mRNA in purified microglia relative to the amount in mixed astrocyte/microglia both standardized to actin determined by the comparative Ct method. Note the log scale, so that bars going up from 1 indicate increases in purified microglia relative to mixed glial cultures, and bars going down from 1 indicate decreases. Each experiment was in triplicate with the number of individual experiments in parentheses. *P ≥ 0.05 compared with mRNA in mixed astrocyte/microglia cultures. Data mean ± S.D. (shown in positive direction only because of the logarithmic scale).
Comparing the relative amounts of purinergic receptor mRNA in microglia and mixed glial cultures using the Comparative Ct Method, the most abundant purinergic receptor mRNA in microglia is P2Y6 followed by P2X4, P2Y12, and P2X7 = P2Y13 (Fig. 4). Because small amounts mRNA encoding other P2Y receptors (all normalized to β-actin) (P2Y1, P2Y2 and P2Y4) and P2X receptors (P2X3, 1 × 10−5 in astrocytes and not detectable in microglia; P2X5, 8 × 10−6 in astrocytes and 8 × 10−8 in microglia) were measured in purified microglia, in this state of activation at least, these receptors are most likely on either astrocytes or oligodendrocytes but not on microglia. P2Y14, which is the UDP-glucose receptor, is probably located on both astrocytes and microglia (1.2 × 10−4 in astrocytes and 1.1 × 10−5 in microglia).
Extracts from cerebral cortex and spinal cord from adult mice were also used for real-time PCR (Fig. 5). Note that the amounts of mRNA for each P2 receptor are in the same order of magnitude as that in mixed astrocyte/microglia cultures. The relative amounts of each receptor are also similar except for P2Y6 which is less abundant in cortex and spinal cord, and P2Y12 which is more abundant in cortex and spinal cord.
Fig. 5. Real-time PCR of P2 receptor mRNA in cerebral cortex and spinal cord.
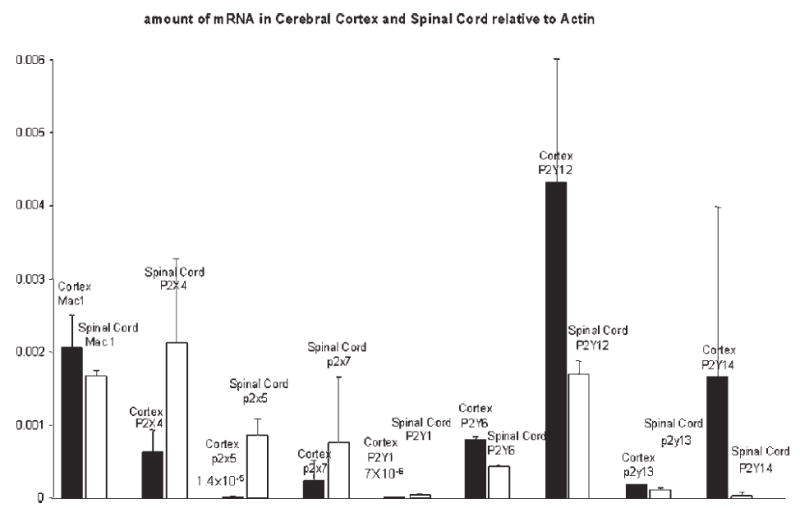
The amount of mRNA in cerebral cortex and spinal cord, relative to actin, from two mice determined by the comparative Ct method. Each primer–probe set was run in triplicate. Note that the relative amount of each P2 receptor is the same magnitude as that in mixed astrocyte/microglia cultures. Data are mean ± S.D.
Calcium dose-response
Microglia plated for either 4 or 24 hours demonstrated rapid, dose-related increases in intracellular free calcium levels in response to ATP. In some dishes, >85% of the microglia responded to 10 μM ATP. On average, 44% of microglia demonstrated a significant increase in intracellular free calcium in response to 50 nM ATP, whereas microglia did not respond to the vehicle alone.
Dose-response curves (Fig. 6) indicate that the intracellular calcium response was most sensitive to 2MeSADP. At 4 hours after plating, EC50 values for each agonist were: 2MeSADP, ~8.6; UDP, ~8.3; UTP, ~8.3; IDP, ~7.8; ADP, ~7.8; and ATP, ~6.2. (−log10 EC50 molar values). At 24 hours after plating, EC50 values for each agonist were: 2MeSADP, ~9.3; UDP, ~7.6; ADP, ~7.5; UTP, ~7.3; ATP, ~6.5; and BZATP, ~5.7. Fitting these data to curves based on single-receptor kinetics revealed good fits for UTP and ATP, and approximate fits for UDP, ADP and 2MeSADP. The sensitivity of the agonists is also indicated by the Kd values derived from the 24-hour curves: 2 MeSADP, 14 nM; ADP, 70 nM; UDP, 70 nM; UTP, 240 nM; ATP, 1.5 μM; and BzATP, 13 μM.
Fig. 6. Dose-response curve for calcium responses to nucleotides from microglia 4 hours and 24 hours after plating.
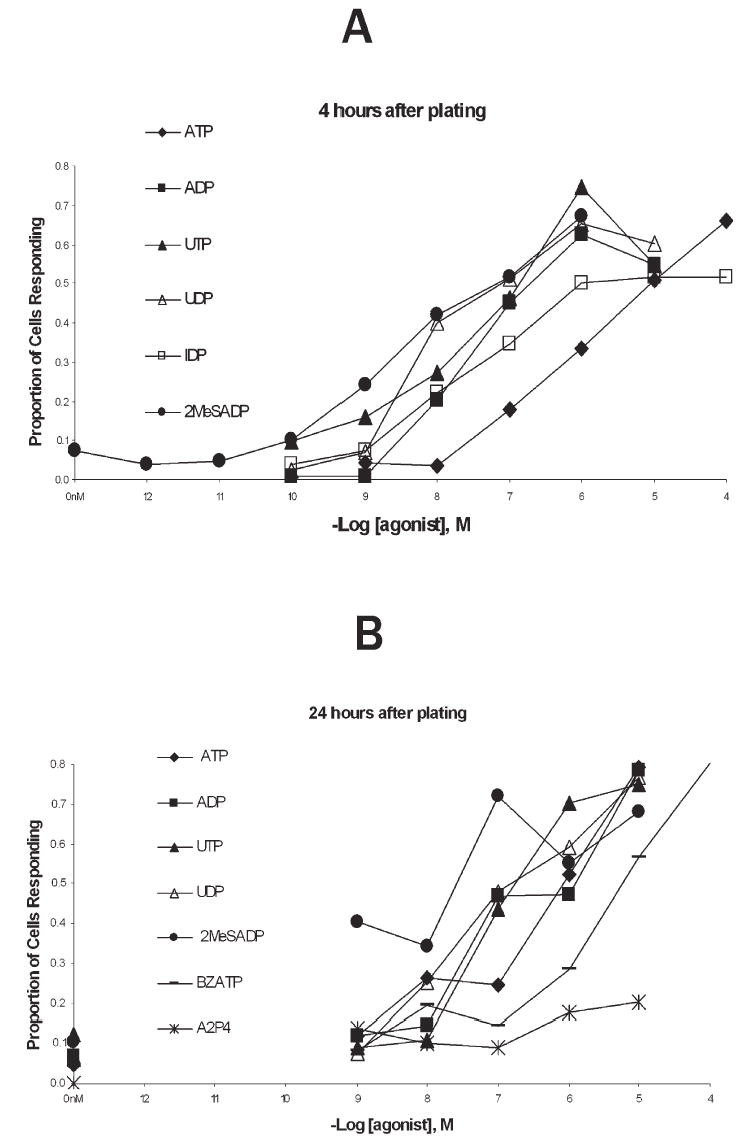
(A) Microglia plated for 4 hours. Each data point obtained from 3–6 separate experiments on different multi-well plates, from different passages for each data point. Nucleotides were applied at the concentrations indicated, as described in the methods. Positive responses were set at fluorescence intensities exceeding the control values by at least two S.D.s for two consecutive frames (see Methods). S.D.s are not shown here for clarity, but ranged from 0.02–0.9. (B) Dose-response curves in microglia plated for 24 hours, plotted on same scale as (A). Note the addition of BZATP and A2P4.
To test the specificity of microglial responses to ATP, we tested other purinergic receptor agonists and agents that cause rapid calcium increases in astrocytes. Adenosine (100 μM) did not evoke calcium increases. These same wells of microglia responded well to application of 2 μM ATP (data not shown). The non-hydrolyzable ADP-like agent, ADPβS (10 μM) caused a rapid calcium increase in the majority of microglia, confirming that ADP is likely to be the active agent when ADP is applied.
Glutamate (1 mM) and KCl (60 mM) caused a gradual increase in intracellular Ca2+ in microglia in some dishes, but <10% of the microglia in these dishes showed any Ca2+ changes. These slow changes are different from the characteristically rapid response to ATP and other purinoceptor agonists.
P2 receptor antagonists
The responses to ADP and UDP indicate involvement of P2Y receptors. This is further supported by data showing that 100 μM PPADS (which blocks most P2X receptors and blocks calcium waves in astrocytes) had little effect on ATP induced calcium increases in microglia (Fig. 7). Conversely, 100 μM suramin, which is a more general blocker of purinergic receptors and is effective at most P2Y receptors, significantly inhibits the calcium response to ATP in microglia.
Fig. 7. Calcium responses evoked by ATP are blocked by suramin but not by PPADS and responses to three nucleotides are reduced by PTx.
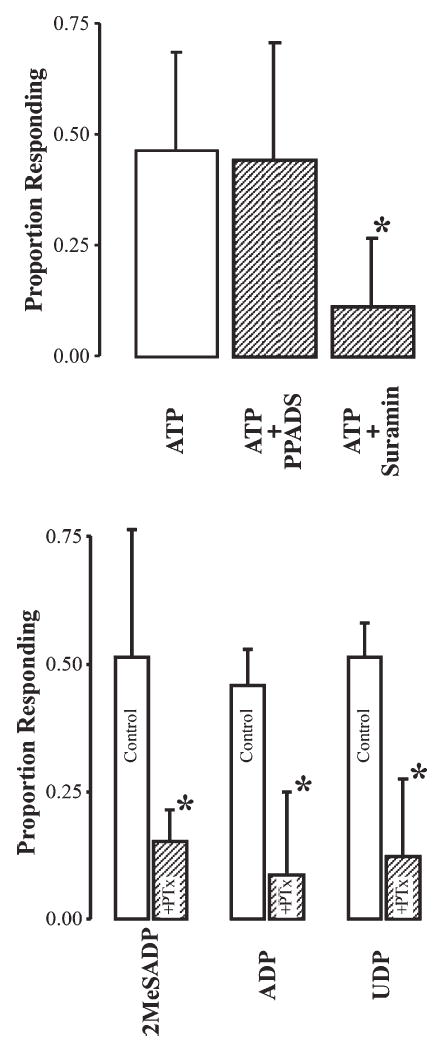
Top: In blocking experiments, either PPADS (100 μM) or suramin (100 μM) were applied for 5 minutes before application of ATP. Then 2 μM ATP + PPADS (n = 9) or 2 μM ATP + suramin (n = 10) were added to evoke responses. *, P<0.001 (t-test, 2-tailed with unequal variance) comparing ATP (n = 11) with ATP + suramin. Bottom: Calcium responses to nucleotides are blocked by PTx (500 ng ml−1) for 1 hour before application of the indicated nucleotides. All nucleotides were applied at 100 nM in observation medium containing 500 ng ml−1 PTx. *, P<0.05 (t-test, 2-tailed with unequal variance) n = 6–7 for each agonist. Data are mean ± S.D.
Additional evidence that P2Y receptors are at least partially involved is the finding that PTx significantly reduces the calcium response to 2MeSADP, ADP and UDP (P<0.05), indicating G-protein mediation of the Ca2+ response and that ionotropic P2X receptors have only a partial role in the responses to these agonists (Fig. 7). It is unlikely that the P2Y1 receptor is involved because mRNA expression in microglia is low and the specific P2Y1 receptor antagonist, MRS 2179 (30 μM) had no effect on the responses induced by 2 MeSADP, ADP and UDP (Fig. 8). However, this dose of MRS 2179 blocks calcium waves in astrocytes, which are thought to be mediated largely by P2Y1 receptors).
Fig. 8. Calcium responses to nucleotides are not blocked by the P2Y1 antagonist MRS 2179: responses to high doses of ATP are blocked by o-ATP a P2X7 antagonist.
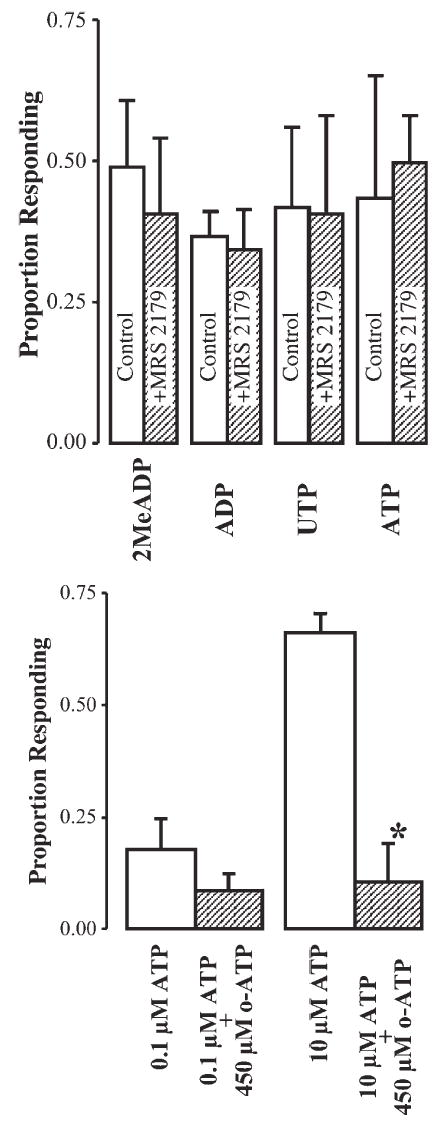
Top: Nucleotides were applied at 100 nM for 2 MeSADP (n = 4), ADP (n = 4) and UTP (n = 4), and 2 μM for ATP (n = 5). In all experiments, MRS 2179 (30 μM) was applied 5 minutes before and included with the nucleotide. Bottom: Effect of 450 μM o-ATP on the proportion of microglia that respond to ATP. Microglia were pre-incubated in o-ATP for 2 hours and ATP added to the culture wells (n = 3 in all experiments). *, P<0.01 (2-tailed, unequal variance t-test). Data are mean ± S.D.
In addition to P2Y receptors, the response to BZATP indicates P2X7 receptor involvement at relatively low concentrations of ATP compared with other reports. This is further indicated by the effects of o-ATP, a specific P2X7 receptor antagonist. As shown in Fig. 8, o-ATP significantly reduces the response to 10 μM ATP, but only slightly (and non-significantly) reduces the response evoked by 100 nM ATP.
This is consistent with the hypothesis that 10 μM ATP activates P2X7 receptors but 100 nM ATP does not.
Shape changes
Each agonists, except UTP and UDP, caused significant shape changes within 5 minutes of application. Shape changes included extension of filopodia, ruffling of the edges, overall flattening of the cells, and obvious rapid movement. In addition, phase contrast was often reduced after application of agonists, which is, apparently, related to the loss of intracellular membranous inclusions. The dose-response curves for shape changes are shown in Fig. 9. The rank order for the potency of the agonists to cause shape changes was similar to that causing Ca2+ increases with 2MeSADP>ADP>ATP. However, both IDP and UTP produced fewer maximal responses than the other agonists, and no responses were observed with UDP. This indicates that P2Y13 (which is normally activated by IDP) and P2Y6 (which is activated strongly by UDP) might play a lesser role in causing shape changes than in inducing rapid calcium changes.
Fig. 9. Shape changes evoked by application of agonists.
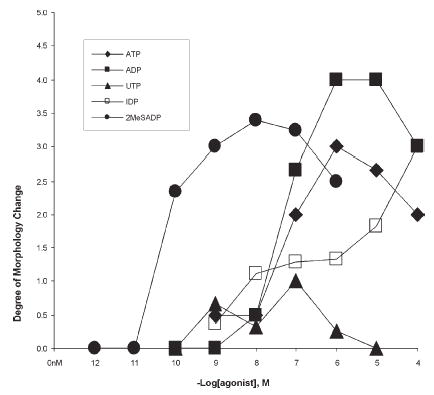
Results of at least three experiments are included in each data point.
CONCLUSIONS
Real-time PCR confirms the presence of mRNA that encodes P2 receptor subtypes and with relative abundance P2Y6>P2X4>P2Y12>P2X7=P2Y13 in mouse microglia in culture.
Full dose-response curves of rapid calcium increases demonstrate that the order of agonist potency is 2MeSADP >UDP=ADP=IDP=UTP>ATP>BzATP, which indicates that each of the above receptors contributes to the rapid calcium responses observed in cultured mouse microglia
P2 receptor agonists cause shape changes at similar doses, which indicates that these effects are caused by activating receptor subtypes similar to those that cause rapid calcium increases in mouse microglia
Real-time PCR shows little, if any, mRNA that encodes P2X3, P2X5, P2Y1, P2Y2, and P2Y4 receptors in mouse microglia.
DISCUSSION
The results presented here predict that both P2Y and P2X receptor subtypes have a role in the activation of microglia by nucleotides that are released from either primary afferents or astrocytes following injury and inflammation that is signaled from the periphery, or locally released by brain or spinal cord injury. We have shown using real-time PCR that the most abundant P2 receptors in microglia are P2Y6, followed by P2X4>P2Y12>P2X7=P2Y13; these pharmacological data also indicate that each of these receptor subtypes might contribute to the rapid calcium increases in microglia evoked by nucleotides.
mRNA expression
The results of this paper place P2Y6, P2Y12, P2Y13, P2X7, and P2X4 receptors in mouse brain microglia, which confirms previous studies (Zhang et al., 2002; Tsuda et al., 2003). In addition, the present data suggest that astrocytes express P2Y1 and P2Y2 receptors (also suggested by Jimenez et al., 2000; Fumagali et al., 2003; Franke et al., 2004). There was very little expression of P2Y4 receptors in mixed cultures, and even less in purified microglia, indicating that either expression is low in astrocytes or expression by cellular contaminants (either oligodendrocytes or neurons) in the mixed glial cultures. Recently, others have also shown that P2Y12 and P2Y13 receptors are expressed in brain (Fumaglia et al., 2004; Hollopeter et al., 2001; Laitinen et al., 2001; Sasaki et al., 2003). Although Bianco et al. 2005 provide evidence that all mammalian P2 receptors are expressed in mouse microglia and that these receptors are differentially expressed in activated compared with resting microglia, our data do not confirm their results. Instead, several of P2 receptor subtypes have little expression in microglia. These differences probably occur because Bianco et al. used an immortalized cell line for some analyses and relied on rtPCR for detection. Although our rtPCR showed similar results, these were refined further by the more quantitative real-time PCR. This latter technique indicates that P2X3, P2X5, P2Y1, P2Y2, and P2Y4 receptors are expressed not by microglia but by other cells in astrocyte/microglia mixed cultures.
Thus, microglia resemble peripheral immune cells in which P2X4, P2Y13 and P2Y6 receptors occur. Interestingly, P2Y12 receptors are expressed highly by platelets, and have been localized to immune cells recently (Jin et al., 1998; Fumaglia et al., 2004; Wang et al., 2004).
Rapid Ca2+ responses
The potent response to 2MeSADP and ADP indicates the involvement of either P2Y12 or P2Y13 receptors, the later of which is emphasized because of the response to IDP (Holopeter et al., 2001; Zhang et al., 2002). The inflection in the 24 hour 2MeSADP curve is consistent and might be caused by the recruitment of additional receptor(s), for example P2Y6 receptors, at micromolar doses. The more prominent inflection at 24 hours might reflect alterations in receptor expression over time, a possibility we are investigating currently. The robust response to UDP also indicates P2Y6 receptor involvement. The response to UTP is puzzling because it is proposed to be most potent at activating P2Y2 and P2Y4 receptors, which are essentially not expressed by microglia (see real-time data). Part of the response to UTP might be caused by hydrolysis of UTP to UDP but, if so, we would expect the response to UTP to be smaller than that to UDP. Others have also observed robust responses to UTP (Möller et al., 2000). The response to A2P4 was variable, and in <20% of the cells, even at the highest doses in microglia plated for either 4 or 24 hours. The lack of a robust response to A2P4 indicates weak involvement of P2X4 and P2Y12 receptors, but might also result from antagonism by A2P4 of the P2Y13 receptor (Marteau et al., 2001; Patel et al., 2001) coupled with agonist action at P2X4 receptors. Although responses to all other agonists were also similar at both 4 hours and 24 hours after plating microglia, with the exception of the response to 2MeSADP, they were somewhat more robust at 4 hours, indicating possible downregulation with time after plating.
The order of effectiveness of purinergic agonists on Ca2+ responses, 2MeSADP>UDP=ADP=IDP=UTP>ATP, is most consistent with activation of P2Y12 receptors, which have been cloned recently. These receptors are most sensitive to 2MeSADP and ADP, and less sensitive to ATP (reviewed by Communi et al., 2001; Foster et al., 2001) (Hollopeter et al., 2001; Nicholas, 2001). However, responsiveness to UDP also indicates a contribution from P2Y6 receptors. Sasaki et al. 2003 also suggested a role for P2Y12 receptors based on rat-brain mRNA expression and in situ hybridization. The sensitivities to ADP and IDP also indicate partial involvement of P2Y13 receptors in the generation of the rapid Ca2+ responses (Fumaglia et al., 2004). Although P2Y1 receptors have a similar pharmacological profile to these agonists (Lazarowski et al., 2001), our data indicate that they probably do not play a role because little P2Y1 mRNA is expressed in microglia, and PPADS and the prototypical P2Y1 receptor antagonist MRS2179 do not block the calcium responses.
The prominence of the response to 2MeSADP, the lack of response to A2P4, and the lack of blockade by PPADS indicate that P2X4 receptors do not dominate the rapid Ca2+ response. However, PTx does not abolish completely the Ca2+ response, and the fairly robust response to ATP indicates a substantial contribution from P2X4 receptors for which mRNA expression is also robust.
The sensitivity to ADP and the lack of response to adenosine rules out adenosine receptors in mediating the rapid Ca2+ responses. The ability of suramin, but not PPADS, to block responses to ATP rules out most of the other P2X receptors as does the partial blockade of responsiveness by PTx. However, these data are consistent with P2Y6, P2Y12 and P2Y13 receptor activation mediating the rapid Ca2+ responses.
Shape changes
It appears that rapid Ca2+responses are not always linked to shape changes and resultant chemotaxis. UTP and UDP activate rapid Ca2+responses readily, but do not cause robust shape changes.
Ca2+ responses to UTP in microglia, as observed in this report, have been demonstrated previously (Möller et al., 2000). However, apparently UTP does not activate the same cellular effects as ATP and ADP such as chemotaxis and the release of interleukin 6 (IL-6) (Norenberg et al., 1997; Honda et al., 2001; Shigemoto-Mogami et al., 2001), which indicates that activating different receptor subtypes has different effects on cell function. Heterogeneity in microglial responses has been reported by Möller et al. 2000 who suggest that different states or populations of microglia express different P2Y receptors. They specifically suggest that expression of P2Y receptors decreases in activated microglia. Of course, the response profiles of the agonists and antagonists applied here might also be explained by microglial-specific effects of these receptor subtypes, splice variants, heterodimerization of more than one receptor subtype, and by the existence of an unidentified P2Y receptor (e.g. Ferrari et al., 1996; Ferrari et al., 1997; Ferrari et al., 1999). For example, cloning of the P2Y14 receptor was reported recently, and Bianco et al. 2005 report the presence of this receptor on microglia, which we have confirmed with real-time PCR (data not shown). However, the P2Y14 receptor is not expected to contribute to the pharmacological profile reported here because it is activated preferentially by glucose compounds.
The pore-forming P2X7 receptor
Our data indicate that the pore-forming P2X7 receptor is expressed by both astrocytes and microglia.
The prototypical P2X7 agonist BzATP evoked a large, rapid Ca2+ increase in microglia, indicating possible activation of P2X7 receptors with higher doses of ATP. The P2X7 antagonist, o-ATP, significantly reduced the ATP responses, and BzATP activated the Ca2+ response at doses consistent with P2X7 receptor activation. The addition of a longer lasting Ca2+ increase at higher doses of ATP also hints at the activation of additional receptors with increasing concentrations of ATP. This is not unexpected given previous reports (Ferrari et al., 1996; Ferrari et al., 1997; Ferrari et al., 1999; Visentin et al., 1999; Hide et al., 2000; Inoue et al., 1998) indicating that doses of ATP as low as 10 μM might activate P2X7 receptors. However, most of the effects that are attributed to P2X7 recetors are thought to occur at millimolar doses. We suggest that activation of the P2X7 receptor at low doses of ATP occurs without pore formation as suggested by Gudipaty et al. 2001 because 100 μM ATP application for one hour does not lead to cell lysis. With higher (millimolar) doses of ATP we observe cell lysis, as observed by others (data not shown).
Functional significance
Microglia are present in large numbers throughout the normal CNS. Normally, they are in a ‘resting’ state in which they have highly ramified, large, non-overlapping dendritic distributions, and express few markers of their immune cell lineage (Pennell and Streit, 1998). They are activated rapidly following either injury or infection of the CNS (e.g. Streit et al., 1999). Very recently, they have also been shown to be activated rapidly in specific regions of the spinal cord and brain that receive inputs from either noxious or inflammatory insults to the periphery, under conditions in which degeneration of spinal cord elements are unlikely (Fu et al., 1999; Fu et al., 2000; Fu et al., 2001; Sweitzer et al., 1999; Raghavendra et al., 2004). It appears that this activation is most likely to be mediated by neural signaling from the peripheral inflammation site to the CNS.
The in vivo mediators of this rapid activation caused by injury outside the CNS are unknown. Many substances released by injury have been shown to cause appropriate changes in microglia, including transforming growth factor α (TGF-α), C5a, EGF, fractalkine and ATP (Yao et al., 1990; Hayashi et al., 1995; Nolte et al., 1996; Nolte et al., 1997; Cross and Woodroofe, 1999; Honda et al., 2001; Chen et al., 2002). One substance that might mediate both neuronally mediated and injury-induced activation is ATP (Zimmermann 1994). ATP has long been known to be released by injury and infection in tissues of all kinds. In addition, ATP is released by presumed nociceptive afferents in the spinal cord (Bardoni et al., 1997).
Following noxious events in the periphery, primary afferent nociceptors might release either ATP or other cytokines in the spinal cord, which result in activation and recruitment of microglia to the vicinity of the released ATP. In addition, astrocytes are activated rapidly by noxious events signaled from the periphery (Garrison et al., 1994; Watkins et al., 2001a; Watkins et al., 2001b). It is possible that activation of astrocytes might also cause the release of ATP, either as individual events or as part of an astrocyte Ca2+ wave, which have been observed in culture and, recently, in brain slices in vitro (Cornell-Bell et al., 1990; Venance et al., 1998; Newman, 2001; Schipke et al., 2001). This additional, astrocyte-derived ATP might further enhance the possibility of activation and recruitment of microglia into the vicinity of the spinal cord where primary afferent nociceptors terminate as well as causing the release of neurally active cytokines by microglia (Ferrari et al., 1996; Ferrari et al., 1997; Inoue et al., 1998; Hide et al., 2000; Sanz and Di Virgilio, 2000; Honda et al., 2001; Shigemoto-Mogami et al., 2001).
The significance of activation and recruitment of microglia in pathological and pain states is unknown presently, but is postulated to be either destructive or protective. Most recently, both contributions have been predicted, depending on when and to what state microglia are activated (Streit et al., 1999). In pain models, activation of microglia correlates with the timing of enhanced, persistent pain. Thus, it is possible that microglia play a role in enhancing pain by participating in a cytokine cascade similar to that mediated in the periphery by macrophages (Fu et al., 2000; Fu et al., 2001). The microglia receptors that contribute to this activation are likely to be both P2X (Tsuda et al., 2003) and P2Y (Wu et al., 2004) receptors. Our hypothesis is that injury and inflammation in the periphery activates nociceptive primary afferents that release ATP in the spinal dorsal horn. This ATP release activates astrocytes and microglia. Astrocytes release more ATP, recruiting more activated microglia. Activated microglia release TNFα, IL-6 and other cytokines, which enhance synaptic transmission of nociceptive inputs in the dorsal horn increasing the nociceptive signal to higher brain centers, resulting in enhanced pain. This hypothesis predicts that P2 receptor antagonists that are either applied to or reach the spinal cord systemically, might be useful in treating pain enhanced by injury and inflammation.
Supplementary Material
Acknowledgments
Supported by NIH grants P01 NS39420 (A.R.L.) and NS37024 (P.B.G.). We thank the University of Utah Genomics core, and Mike Klein and the University of Utah real-time PCR facility for help with the real-time PCR data reported here; Sheryl Scott, Guoying Wang and S.B. Kater for discussions about these experiments and comments on the manuscript; Kathy Charters, Helen Willcockson, Wendy Heck, Bonnie Blake and Ryan Price for technical assistance; and Ben Chan for help with analysis programs.
Footnotes
Movies showing shope changes caused by ATP and calcium increases in microglia are included with the online version of this article at http://journals.cambridge.org/jid-NGB.
References
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Research. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, Macdermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, et al. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Research Reviews. 2005;48:144–156. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Chen S, Luo D, Streit WJ, Harrison JK. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. Journal of Neuroimmunology. 2002;133:46–55. doi: 10.1016/s0165-5728(02)00354-5. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Experimental Neurology. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochemical & Biophysical Research Communications. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, Brézillon S, Lannoy V, Parmentier M, et al. Identification of a novel human ADP receptor coupled to Gi. Journal of Biological Chemistry. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cross AK, Woodroofe MN. Chemokines induce migration and changes in actin polymerization in adult rat brain microglia and a human fetal microglial cell line in vitro. Journal of Neuroscience Research. 1999;55:17–23. doi: 10.1002/(SICI)1097-4547(19990101)55:1<17::AID-JNR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. Journal of Immunology. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. Journal of Biological Chemistry. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. Journal of Immunology. 1996;156:1531–1539. [PubMed] [Google Scholar]
- Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C, et al. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. Journal of Clinical Investigation. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Long-lasting inflammation and hyperalgesia after subcutaneous formalin injection into the rat hindpaw. Journal of Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Research. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, et al. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43:218–03. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana C, Abbracchio M. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochemical Pharmacology. 2004;68:113–124. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Molecular Pharmacology. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Experimental Neurology. 1994;129:237–243. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annual Review of Neuroscience. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Gudipaty L, Humphreys BD, Buell G, Dubyak GR. Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. American Journal of Physiology. Cell Physiology. 2001;280:C943–C953. doi: 10.1152/ajpcell.2001.280.4.C943. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. Journal of Neuroscience. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. Federation of American Societies for Experimental Biology Journal. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proceedings of the National Academy of Sciences of the U.S.A. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. Journal of Neuroimmunology. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. Journal of Neurochemistry. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. Journal of Neuroscience. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, Deleo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine. 2001;26:2073–2079. doi: 10.1097/00007632-200110010-00005. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, et al. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. British Journal of Pharmacology. 1998;123:1304–1310. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. Journal of Pharmacological Science. 2004;94:112–114. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Castro E, Communi D, Boeynaems J, Delicado E, Miras-Portugal M. Coexpression of several types of metabotropic nucleotide receptors in single cerebellar astrocytes. Journal of Neurochemistry. 2000;75:2071–2079. doi: 10.1046/j.1471-4159.2000.0752071.x. [DOI] [PubMed] [Google Scholar]
- Jin J, Dasari V, Sistare F, Kunapuli S. Distribution of P2Y receptor subtypes on haematopoietic cells. British Journal of Pharmacology. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in Neuroscience. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Laitinen JT, Uri A, Raidaru G, Miettinen R. [35S]GTPgS autoradiography reveals a wide distribution of Gi/o-linked ADP receptors in the nervous system: close similarities with the platelet P2YADP receptor. Journal of Neurochemistry. 2001;77:505–518. doi: 10.1046/j.1471-4159.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Rochelle LG, O’Neal WK, Ribeiro CM, Grubb BR, Zhang V, et al. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. Journal of Pharmacology and Experimental Therapeutics. 2001;297:43–49. [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, et al. Pharmacological characterization of the human P2Y13 receptor. Molecular Pharmacology. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- Möller T, Kann O, Verkhratsky A, Kettenmann H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Research. 2000;853:49–59. doi: 10.1016/s0006-8993(99)02244-1. [DOI] [PubMed] [Google Scholar]
- Möller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. Journal of Neuroscience. 1997;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. Journal of Neuroscience. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RA. Identification of the P2Y12 receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides. Molecular Pharmacology. 2001;60:416–420. [PubMed] [Google Scholar]
- Nolte C, Kirchhoff F, Kettenmann H. Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. European Journal of Neuroscience. 1997;9:1690–1698. doi: 10.1111/j.1460-9568.1997.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Nolte C, Möller T, Walter T, Kettenmann H. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience. 1996;73:1091–1107. doi: 10.1016/0306-4522(96)00106-6. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Cordes A, Blohbaum G, Frohlich R, Illes P. Coexistence of purino- and pyrimidinoceptors on activated rat microglial cells. British Journal of Pharmacology. 1997;121:1087–1098. doi: 10.1038/sj.bjp.0701241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Barnes A, Camacho J, Paterson C, Boughtflower R, Cousens D, et al. Activity of diadenosine polyphosphates at P2Y receptors stably expressed in 1321N1 cells. European Journal of Pharmacology. 2001;430:203–210. doi: 10.1016/s0014-2999(01)01401-7. [DOI] [PubMed] [Google Scholar]
- Pennell NA, Streit WJ. Tracing of fluoro-gold prelabeled microglia injected into the adult rat brain. Glia. 1998;23:84–88. [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of Neuroscience. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Progress in Neurobiology. 1993;41:125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. Journal of Immunology. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. Federation of American Societies for Experimental Biology Journal. 2001;15:1270–1272. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. Journal of Neurochemistry. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Progress Neurobiology. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. Journal of Neurochemistry. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, Deleo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Research. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Current Opinion in Immunology. 2002;1:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- Venance L, Premont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. Journal of Physiology (London) 1998;510:429–440. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. Journal of Immunology. 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- Visentin S, Renzi M, Frank C, Greco A, Levi G. Two different ionotropic receptors are activated by ATP in rat microglia. Journal of Physiology (London) 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. Journal of Neuroscience. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jacobson S, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BioMed Central Immunology. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in Neuroscience. 2001a;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001b;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, Deleo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. Journal of Comparative Neurology. 2001;439:127–139. [PubMed] [Google Scholar]
- Wu Y, Willcockson HH, Maixner W, Light AR. Suramin inhibits spinal cord microglia activation and long-term hyperalgesia induced by formalin injection. Journal of Pain. 2004;5:48–55. doi: 10.1016/j.jpain.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Yao J, Harvath L, Gilbert DL, Colton CA. Chemotaxis by a CNS macrophage, the microglia. Journal of Neuroscience Research. 1990;27:36–42. doi: 10.1002/jnr.490270106. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, et al. P2Y13: identification and characterization of a novel Gαi-coupled ADP receptor from human and mouse. The Journal of Pharmacology and Experimental Therapeutics. 2002;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signaling via ATP in the nervous system. Trends in Neuroscience. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


