Abstract
Williams-Beuren syndrome (WBS), caused by a heterozygous deletion at 7q11.23, represents a model for studying hypertension, the leading risk factor for mortality worldwide, in a genetically determined disorder. Haploinsufficiency at the elastin gene is known to lead to the vascular stenoses in WBS and is also thought to predispose to hypertension, present in ∼50% of patients. Detailed clinical and molecular characterization of 96 patients with WBS was performed to explore clinical-molecular correlations. Deletion breakpoints were precisely defined and were found to result in variability at two genes, NCF1 and GTF2IRD2. Hypertension was significantly less prevalent in patients with WBS who had the deletion that included NCF1 (P=.02), a gene coding for the p47phox subunit of the NADPH oxidase. Decreased p47phox protein levels, decreased superoxide anion production, and lower protein nitrotyrosination were all observed in cell lines from patients hemizygous at NCF1. Our results indicate that the loss of a functional copy of NCF1 protects a proportion of patients with WBS against hypertension, likely through a lifelong reduced angiotensin II–mediated oxidative stress. Therefore, antioxidant therapy that reduces NADPH oxidase activity might have a potential benefit in identifiable patients with WBS in whom serious complications related to hypertension have been reported, as well as in forms of essential hypertension mediated by a similar pathogenic mechanism.
Williams-Beuren syndrome (WBS [MIM 194050]) is a neurodevelopmental disorder with multisystemic manifestations and a prevalence in newborns that ranges between 1/7,500 and 1/20,000 (Greenberg 1990; Stromme et al. 2002). The WBS phenotype includes distinctive facial features; vascular stenoses, most notably supravalvar aortic stenosis (SVAS); global cognitive deficit, with an asymmetric neurobehavioral profile; and transient hypercalcemia of infancy. WBS is caused by a segmental aneusomy of 1.55–1.83 Mb at chromosomal band 7q11.23, which includes ELN (coding for elastin [MIM 130160]) and 25–27 additional genes (Francke 1999; Peoples et al. 2000; Pérez Jurado 2003). The WBS deletion is mediated by nonallelic homologous recombination (NAHR) between regional segmental duplications or low-copy repeats (LCRs) that flank the region. Each of the LCR blocks that mediate most deletions, called “blocks B,” contain three transcription units, which are either functional genes or pseudogenes, depending on their location (Valero et al. 2000). GTF2I (general transcription factor II-I [MIM 601679]) and NCF1 (neutrophilic cytosolic factor 1 [MIM 608512]) have been shown to be functional only from a single locus located in the medial block B (Bm) (Gorlach et al. 1997; Pérez Jurado et al. 1998), whereas the other two copies located in the centromeric (Bc) and telomeric (Bt) blocks are pseudogenes with truncating mutations. The third transcription unit, GTF2IRD2 (alpha [MIM 608899] and beta [MIM 608900]), which codes for a protein of unknown function but related to GTF2I, appears to be functional in two of the three copies: those located in Bm and Bt (Tipney et al. 2004; Antonell et al. 2005). Despite the fact that the great majority of patients have deletions very similar in size, the WBS phenotype is highly variable, and the molecular bases of this variability are still unknown. Hemizygosity at ELN is known to be the cause of the vascular stenoses that lead to clinically recognized cardiovascular involvement in as many as 75% of patients and is also thought to predispose to hypertension. Additional candidates for involvement in several aspects of the WBS phenotype have been proposed on the basis of clinical-molecular correlations in patients with partial phenotypes and smaller deletions (Hirota et al. 2003; Morris et al. 2003; Pérez Jurado 2003; Tassabehji 2003; Howald et al. 2005; Tassabehji et al. 2005). However, definitive pathogenetic implication of the genes in the deletion or flanking regions, other than ELN, is still uncertain.
Hypertension is the leading risk factor for mortality worldwide, affecting 26% of the adult population overall, and these numbers are predicted to increase by ∼60% in the next 20 years (Kearney et al. 2005). Understanding the pathogenic mechanisms involved in this condition is one of the highest priorities for public health, to better diagnose, treat, and prevent the disorder. There are multiple genetic and environmental factors contributing to human hypertension, and a role for oxidative stress seems to be somehow present in most forms (Touyz 2005). WBS represents a model for studying the pathogenic mechanism of hypertension in a genetically determined disorder. Interestingly, the hypertension found in ∼50% of young adults with WBS is not often associated with visible vascular stenoses of their descending aortas or renal arteries on abdominal Doppler ultrasound, a fact that speaks against vascular narrowing alone as the determinant factor for hypertension (Cherniske et al. 2004). Thus, high blood pressure in WBS appears to fall in the category of essential hypertension with no well-defined structural cause, since anatomic renovascular hypertension is a very infrequent finding.
We have characterized the clinical features of 96 patients with WBS and have performed fine mapping of the deletions, to investigate whether variation in the breakpoint may affect different genes implicated in this variable phenotype. We have determined that the occurrence of hypertension in WBS is not correlated at all with the severity of the cardiovascular lesions. Instead, hypertension occurs with significantly lower frequency in patients whose deletion includes a functional copy of the gene NCF1, which encodes the p47phox subunit of the NADPH oxidase (NOX) complex. Therefore, for patients with WBS, hemizygosity for NCF1 is a protective factor against hypertension. This finding implicates oxidative stress directly in the pathogenesis of hypertension in WBS and suggests that there is a potential role for antioxidant pharmacological intervention to prevent its development in WBS, as well as other forms of hypertension mediated by a similar mechanism.
Material and Methods
Subjects
Major clinical characteristics were analyzed in 96 patients with the phenotype of sporadic WBS and a confirmed deletion at 7q11.23 who were referred from multiple clinical centers in Spain. Patients’ anthropometrical measurements were compared with normal and WBS standards (Hall et al. 1989; Partsch et al. 1999). All patients were evaluated using a similar protocol for data collection and physical exam. Blood pressure was taken during morning hours, to avoid any factor that could increase anxiety, and on at least two separate occasions. For this study, hypertension was defined as blood pressure measurements >90th percentile for age and sex (Horan 1987).
Molecular Characterization of the Deletions
PCR analyses of single- and multiple-copy microsatellites were performed, to define the size and parental origin of the deletion; genotyping of paralogous sequence variants, also called “site-specific nucleotides” (SSNs), was done thereafter to map the exact breakpoints of the deletion within block B. PCR conditions and oligonucleotides used for the analyses were as described in detail elsewhere (Bayés et al. 2003).
Significance of clinical-molecular correlations was analyzed using the statistical package SPSS 11.5. Specifically, the χ2 test, the Fisher’s exact test, the Student's t test, and logistic regression were used for the analyses, according to the characteristics of each variable. Significance level was established as P<.05.
Determination of NCF1 Copy Number
The NCF1 gene can be distinguished from its pseudogenes by the presence of a 2-bp (GT) deletion at the beginning of exon 2 in the pseudogene copies. PCR reactions were performed with 50 ng of genomic DNA, PCR buffer, 1.5 mM MgCl2, 1.25 mM of each dNTP, 5 pmol of each primer (one labeled with HEX fluorochrome), and 0.25 units of Taq polymerase (Ecogen) on a GeneAmp System 9700 thermocycler (Applied Biosystems) with the following parameters: a denaturing step (5 min at 94°C), 27 denaturing-annealing-extension cycles (at 94°C, 55°C, and 72°C, for 40 s each), and a final extension (7 min at 72°C). Genotyping was performed on an ABI 3100 sequencer, and results were analyzed using the Genescan 3.1 software (Applied Biosystems). Pseudogene/gene ratio quantification was performed by comparing the height of the peaks corresponding to each variant.
Population Study of Essential Hypertension
A population sample composed of individuals randomly selected from cross-sectional studies in the Spanish province of Girona, to establish the prevalence of cardiovascular factors in the region (Masia et al. 1998), was also studied for the NCF1 gene:pseudogene variant. Only strictly normotensive adult subjects (diastolic blood pressure <80 mmHg and systolic blood pressure <135 mmHg; n=68) and definitely hypertensive subjects (diastolic blood pressure >90 mmHg and systolic blood pressure >150 mmHg; n=68) were considered.
Cell Lines
Epstein-Barr–transformed lymphoblastoid cell lines from individuals with different NCF1 gene-copy number were grown in RPMI 1640 medium plus 10% fetal calf serum and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). Culture conditions were 37°C and 5% CO2. Cells were used for the experiments while growing in exponential phase. Cell viability was measured by using the trypan-blue exclusion test, showing a viability of 98%.
Measurement of NOX Activity
Superoxide anion produced during the respiratory burst by the NOX activation was evaluated using the reduction of nitroblue tetrazolium (NBT) assay (SIGMA), which was performed as follows. Aliquots of 250 μl of lymphoblastoid cells (10×106/ml) were mixed with 250 μl of NBT (1 mg/ml) in Hank’s balanced salt solution (HBSS) (Invitrogen-GIBCO). A dose-response curve, with phorbol myristate acetate (PMA) to stimulate NOX, determined that 1 μM PMA was suitable to induce a positive response. Unstimulated cells were treated with HBSS. After 60 min of incubation at 37°C, the reaction was stopped with 0.5 M HCl, and cells were centrifuged. Supernatants were discarded, and the reduced NBT was extracted with dioxan. Supernatant absorbance at 525 nm was determined in an Ultrospec 2100-pro spectrophotometer (Amersham Biosciences). Experiments were performed in duplicate. Data are expressed as percentages, with the assumption that unstimulated cells have 100% NOX activity. Data were evaluated statistically by use of the Student's t test, with a significance level of P<.05.
Western-Blot Analysis
Cells were lysed and protein concentration was determined (Bio-Rad). Forty μg of proteins per sample was run in 10% SDS-PAGE gel for 2 h at 150 V and was transferred onto a nitrocellulose membrane. Membranes were incubated with a 1:500 mouse antibody (Ab) anti-p47phox (BD Biosciences) and then with 1:2,000 anti-mouse horseradish peroxidase-linked Ab (Amersham Biosciences). Loading reference was performed by use of a 1:5,000 mouse anti–α-tubulin Ab (SIGMA). Proteins were detected by an enhanced chemiluminescence kit (Pierce). Bands were quantified with the Scion Image program (Scion Corporation). The ratio between p47phox and α-tubulin was calculated to normalize to the amount of protein loaded per lane.
Immunocytochemistry for p47phox and Detection of Nitrotyrosination
Approximately 7.5×104 lymphoblastoid cells were attached on 1% poly-l-lysine–coated coverslips by cytospinning. Cells were fixed and incubated for 2 h at room temperature with 1:300 mouse anti-p47phox monoclonal Ab (BD Biosciences) or 1:400 anti-nitrotyrosine polyclonal Ab (Molecular Probes), followed by incubation with 1:500 Alexa fluor 488 goat anti-mouse or anti-rabbit Ab (Molecular Probes) for 1 h at room temperature. Digital images were taken with a Leica TCS SP confocal microscope and were analyzed with Leica confocal software.
Results
Clinical and Molecular Characterization
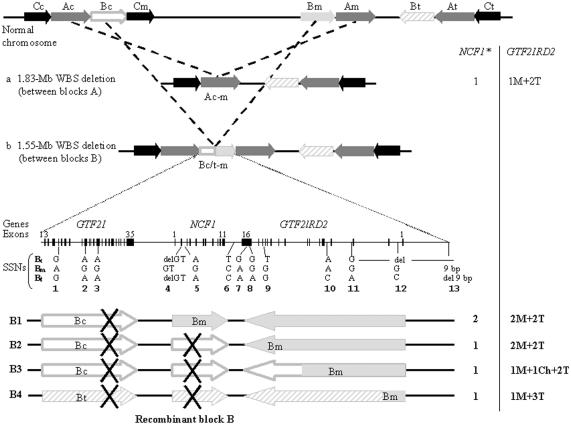
The clinical features of 96 Spanish patients with classic WBS, with a mean age of 8.8 years (range 0–28 years), are shown in table 1. Molecular studies identified a total of 88 patients with a 1.55-Mb deletion, mediated by NAHR between blocks B (fig. 1B), whereas 8 subjects had a larger (1.83-Mb) deletion, mediated by NAHR between blocks A (fig. 1A). Of the deletions, 53% occurred in the maternal chromosome, and 47% in the paternal one. Data at SSNs 8, 10, and 11 permitted inference that the transmitting progenitor was a carrier of an inverted chromosome in 25% of cases, as reported elsewhere (Bayés et al. 2003). The analysis of additional SSNs allowed us to precisely define the breakpoints between two SSNs in the recombinant chromosome of each patient and thus to identify the number of potentially functional copies of NCF1 and GTF2IRD2 (fig. 1 and table 2).
Table 1.
Clinical Features of 96 Patients with WBS
| Clinical Feature | No. with Feature/ No. Assessed |
Percentage |
| Sex: | ||
| Male | 51/96 | 53 |
| Female | 45/96 | 47 |
| Gestational age >41 wks | 28/88 | 32 |
| Birth weight <10th percentile | 48/92 | 52 |
| Height <3rd percentile | 51/83 | 61 |
| Head circumference <10th percentile | 27/66 | 41 |
| WBS facies | 96/96 | 100 |
| WBS personality | 96/96 | 100 |
| Hyperacusis | 73/76 | 96 |
| Cardiovascular lesion | 63/96 | 66 |
| SVAS | 42/96 | 44 |
| PPS | 30/96 | 32 |
| Hypertension: | 26/57 | 46 |
| Systolic | 25/57 | 44 |
| Diastolic | 15/57 | 26 |
| Hypercalcemia | 10/63 | 16 |
| Gastrointestinal symptoms | 78/88 | 89 |
| Urinary tract anomalies | 13/60 | 22 |
| Psychiatric problems | 22/60 | 37 |
| Muscle/skeletal problems | 37/63 | 59 |
Figure 1.
Schematic representation of the common deletions associated with WBS and the characterization of deletion breakpoints. Top, Scheme of the 7q11.23 genomic region, with the large blocks of segmental duplications represented by black (block C), dark gray (block A), and light gray (block B) arrows indicating their relative orientation. The three blocks B are differentiated by unblackened (Bc), blackened (Bm), and diagonally striped (Bt) light gray arrows. The two major types of deletion that cause WBS occur through NAHR mediated by blocks of segmental duplications that are located in tandem, either blocks Ac and Am (a) or blocks Bc/t and Bm (b). Bottom, Genomic structure and resulting recombinant block B in patients with the 1.55-Mb deletion. The schematic representation of the entire block with its gene content (numbered exons of GTF2I, NCF1, and GTF2IRD2) is shown. The location of the multiple SSNs analyzed in this study (with the nucleotide corresponding to each of the locations Bc, Bm, and Bt) is drawn below (numbered as in the work of Bayés et al. [2003]). The resulting chromosomes, depending on deletion breakpoints, are shown with arrows corresponding to functional genes or pseudogenes (marked with an X) indicating their transcriptional direction. B1, Deletion proximal to the NCF1 gene that does not affect the functional copies of GTF2IRD2. B2, Deletion causing the loss of NCF1 but not GTF2IRD2. B3, Loss of NCF1 and breakpoint within GTF2IRD2 creating a medial-centromeric chimeric copy of the gene. B4, Inversion-mediated deletion in which the recombinant chromosome loses NCF1 and a medial copy of GTF2IRD2 but gains a telomeric copy of GTF2IRD2. The numbers of functional copies of NCF1 and GTF2IRD2 corresponding to each rearrangement are displayed at the right of the figure. The asterisk (*) indicates that the possibility of additional NCF1 copies due to polymorphism is not shown in the figure.
Table 2.
Molecular Variants in 96 Patients with WBS
| Molecular Variant | No. (%) of Subjects |
| Parental origin of the deletion: | |
| Maternal | 48 (50) |
| Paternal | 43 (45) |
| Unknown | 5 (5) |
| Inversion-mediated deletion: | |
| Yes | 24 (25) |
| No | 72 (75) |
| Deletion size: | |
| 1.55 Mb (blocks B) | 88 (92) |
| 1.83 Mb (blocks A) | 8 (8) |
| “Functional” copies of NCF1: | |
| 1 | 42 (44) |
| 2 | 41 (43) |
| 3 | 12 (12) |
| 4 | 1 (1) |
| “Functional” copies of GTF2IRD2: | |
| 1M+3T | 24 (25) |
| 2M+2T | 53 (55) |
| 1M+1Ch+2T | 11 (12) |
| 1M+2T | 8 (8) |
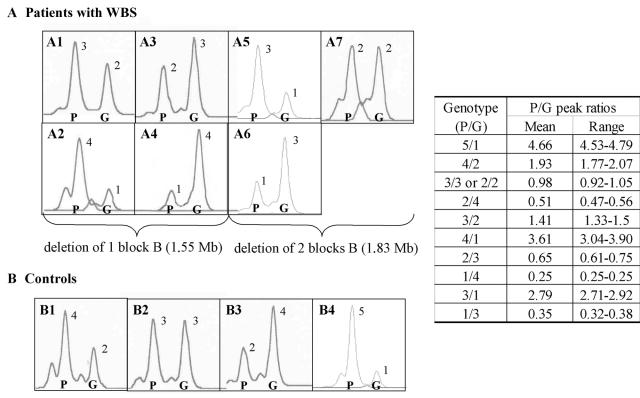
At NCF1, we and others have reported a polymorphism, likely generated by gene conversion between the gene and pseudogenes, that results in the presence of either three (∼15%) or four (∼1%) gene-type copies in the normal population (Heyworth et al. 2002; Bayés et al. 2003). Dependent on the site of the deletion and the presence or absence of this polymorphism, patients with WBS display one, two, three, or four NCF1 gene-type copies (table 2). Representative results of NCF1 genotypes in patients with WBS and control individuals are shown in figure 2.
Figure 2.
Representative genotypes at exon 2 of NCF1 of individuals with different pseudogene/gene copy ratios. Each square includes a genotype with two peaks corresponding to the PCR amplicons of the NCF1 pseudogene (P) and gene (G) copies from several individuals with WBS with 1.55-Mb (A1–A4) or 1.83-Mb (A5–A7) deletions as well as several controls (B1–B3 and B4, a carrier of autosomal recessive chronic granulomatous disease). The predicted number of NCF1 pseudogene and gene copies is shown above each peak. The table at the right displays the actual P/G peak ratio values (mean and range) obtained for each predicted genotype.
At GTF2IRD2, several relevant molecular variants could also be defined, as shown in figure 1. Their frequencies are shown in table 2.
Clinical-Molecular Correlations
Since these 96 patients have a confirmed deletion of the WBS region in band 7q11.23, the frequency of each of the major clinical features listed in table 1 can be used to define the WBS characteristic phenotype and to understand its variability. Some features were age dependent, such as most skeletal problems (spine curves), which are present in over half (59%) of patients and appear after the 1st decade of life (P=.01; χ2 test). Although global cardiovascular involvement was not age dependent, peripheral pulmonic stenosis (PPS) was significantly more frequent in younger patients, who received diagnoses during the 1st decade of life (P=.009; χ2 test). Only cardiac involvement was sex dependent; it was clearly more frequent in males (P=.02; Fisher’s exact test). Except for the fact that microcephaly was more frequent in patients with maternal deletions (P=.01; Fisher’s exact test), no association with parental origin of the deletions was found with other features. However, there was a nonsignificant trend for paternal chromosome deletions to be more frequent in males and for maternal chromosome deletions to be more frequent in females (P=.09; Fisher’s exact test).
No significant associations were found among the different clinical variables. Specifically, no significant associations were found between any subtype or degree of severity of cardiovascular involvement and the presence of hypertension, neither for systolic nor for diastolic hypertension. Patient ages (mean ± SD, 9.7±6.4 years with hypertension vs. 10.8±7.2 years without hypertension), body weights for age, and reported diet and physical activities were not significantly different between the groups with and without hypertension. None of the patients with WBS were smokers. Only nine patients were receiving treatment for established high blood pressure at the time of the evaluation.
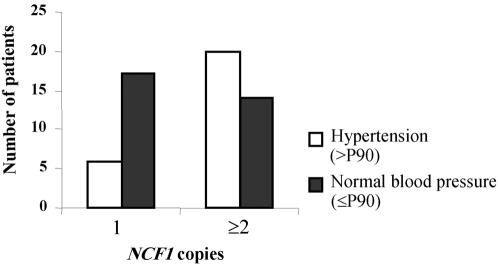
With respect to the different molecular variants, hypertension was found with significantly different prevalence, depending on the number of copies of the functional NCF1 gene. Hypertension was much less frequent in patients with a single functional copy of NCF1 than in the rest of the cohort with two or more copies (P=.02; Fisher’s exact test) (fig. 3). The presence of a third or fourth NCF1 copy due to polymorphism was not significantly associated with an increase in the prevalence of hypertension, when compared with the most frequent two-copy variant (P=.29; Fisher’s exact test). The presence of more than one copy of NCF1 appears to be associated with an increased risk for hypertension, with a raw odds ratio of 4.04 (95% CI 1.28–12.84).
Figure 3.
Significant association between hypertension (>P90 = blood pressure >90th percentile) and NCF1 copy number. The histogram clearly shows that patients with a single copy of NCF1 are overrepresented in the group with normal blood pressure (⩽P90 = blood pressure ⩽90th percentile), whereas patients with two or three NCF1 copies cluster in the hypertension group (P=.02; Fisher’s exact test).
Among the four variants of GTF2IRD2 (table 2), the variant 2M+2T, which is associated with no deletion of NCF1, is thus significantly associated with a higher risk of hypertension (P=.01; Fisher’s exact test). Multiple linear regression analysis to study the impact on hypertension of several variables (sex, age [in decades], parental origin of the deletion, cardiovascular involvement, and NCF1 copy number) showed a significant adjusted odds ratio for only NCF1 copy number (odds ratio of 3.62; 95% CI 1.13–11.6).
No other significant associations were found among the remaining clinical and molecular variables.
Association Studies of Essential Hypertension with the NCF1 Copy-Number Polymorphism
To define whether the NCF1 gene copy-number variant may have a role in essential hypertension, we genotyped 68 adult patients with essential hypertension and 68 age- and sex-matched normotensive controls. The genotype frequencies were not significantly different between both groups. In the group with essential hypertension, 56 (82.35%) had two NCF1 copies, 11 (16.17%) had three copies, and 1 (1.47%) had four copies, whereas, among the normotensive control individuals (n=68), 57 (83.82%) showed two copies, 10 (14.7%) showed three copies, and 1 (1.47%) had four copies (P=.53; χ2 test).
Functional and Expression Studies of Patients with WBS and Control Individuals with One, Two, or Three NCF1 Gene-Type Copies
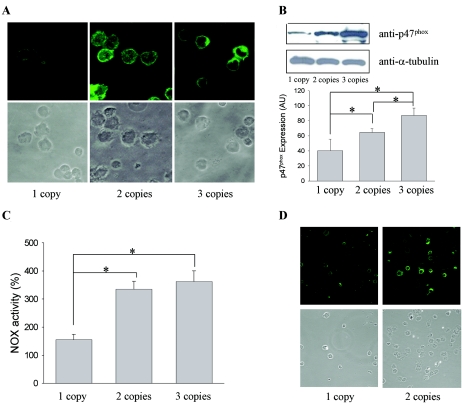
Immunodetection of p47phox in lymphoblastoid cell lines showed a significantly reduced expression of the protein in cell lines with only one NCF1 gene-type copy, compared with those with two or three copies (fig. 4A). This finding was confirmed by western-blot analysis, which showed significantly lower levels of p47phox protein (P=.04; Student's t test) in cells from patients hemizygous for the NCF1 gene (mean result ± SE 40.28±15.3 arbitrary units [Scion Image software]) than in cells with two gene-type copies (64.5±4.9). There was also a significant increase of protein expression in cell lines with three copies (87.1±8.9) (P=.02; Student's t test) (fig. 4B).
Figure 4.
Expression and functional analyses of NCF1 copy number in cell lines. A, Immunodetection of p47phox by cytochemistry. B, Western-blot analysis and quantification of expression levels of p47phox versus α-tubulin. Mean values for expression levels in arbitrary units (AU) for the different groups with 1, 2, and 3 gene-type copies of NCF1 reflect an increase in protein expression. An asterisk (*) indicates significant difference (P<.05). C, Reduction of NBT, shown as the percentage of NOX activation after PMA treatment, with respect to basal conditions for each cell line. Mean values are 155.55, 335.31, and 362.42 for the groups with one, two, and three copies, respectively. The activation is significantly lower when there is one functional copy of the gene, in comparison with two copies (P=.006; t test) and three copies (P=.01; t test). An asterisk (*) indicates significant difference (P<.05). D, Detection of nitrotyrosination by immunocytochemistry.
We also tested NBT reduction in cell lines from patients with WBS who were hemizygous at NCF1 and in control cell lines with two and three NCF1 gene-type copies. We observed a significant decrease in NBT reduction for patients with only one gene-type copy of NCF1, when compared with individuals with two (P=.007; Student's t test) or three (P=.012; Student's t test) gene-type copies. However, there was no significant difference (P=.29; Student's t test) between samples with two and three gene-type copies (fig. 4C).
Finally, presuming that a consequence of NCF1 haploinsufficiency could be less activity of the NOX complex and higher bioavailability of nitric oxide, we compared basal levels of protein nitrotyrosination between cell lines by immunocytochemistry. Nitrotyrosination levels were high, on average, although highly variable among cells with two NCF1 copies, whereas cells with only one copy showed a more homogeneous pattern, with overall lower levels of nitrotyrosination (fig. 4D). Protein nitrotyrosination reflects the effect on proteins of the highly reactive peroxynitrite, which is formed by the reaction of superoxide and nitric oxide.
Discussion
We reviewed the major clinical manifestations of 96 patients with WBS and defined, in detail, their molecular lesion, including parental origin of the deletions and fine mapping of the deletion breakpoints. As for other published series, the WBS phenotype is complex and variable, and some clinical manifestations are age dependent. The molecular bases of this phenotypic variability remain unknown. Differences in the size or location of the breakpoints of the deleted interval, genetic variants in the nondeleted allele, subtle imprinting effects, and the genetic background and environmental factors may all contribute to the variable expression of the phenotype. Partial deletions of the interval have been identified in a few atypical patients and have revealed a potential contribution to the phenotype for the specific genes for which the studied families are hemizygous (Hirota et al. 2003; Morris et al. 2003; Pérez Jurado 2003; Tassabehji 2003; Howald et al. 2005). However, no cis- or trans-acting factors have yet been implicated in the etiology of phenotypic differences in patients with common deletions that appear identical in size.
We found interesting associations among the explored variables, such as cardiovascular stenoses being more frequent in males, as reported elsewhere (Sadler et al. 2001), and microcephaly occurring more frequently with maternally inherited deletions. Correlation of shorter stature and microcephaly with maternally inherited deletions had been suggested in the past but not confirmed in other series (Pérez Jurado et al. 1996; Wang et al. 1999). The recent finding of preferential hotspots for NAHR in male and female meiosis (L.A.P.J., unpublished data) indicates that parental origin of the deletion should always be considered in association with detailed mapping of deletion breakpoints. When that is considered, our results point again to the possibility that genomic imprinting that affects genes in the region might play a role in determining head size. This finding deserves to be explored in further depth and in larger series as a potential determinant of phenotypic variation.
The most remarkable finding of this study concerns hypertension, an important yet inconsistent feature of WBS. High blood pressure is the only explored clinical variable strongly associated with the location of the deletion breakpoint, such that its prevalence among patients with WBS is significantly increased when NCF1 is not included in the deletion. A significant association is also found for hypertension and some GTF2IRD2 variants. However, although little information is yet available about GTF2IRD2 function, our data suggest that it is less likely that GTF2IRD2 is the gene responsible for the variable blood pressure. Individuals with WBS who have GTF2IRD2 variants that result in deletion of one medial copy (1M+2T) or a chimeric copy (1M+1Ch+2T) are predicted to carry hypomorphic alleles. The variant 2T+2M implies that the WBS chromosome maintains both functional GTF2IRD2 gene copies, as in controls, and consequently should be considered normofunctional, whereas the variant 1M+3T could be either normofunctional or even hypermorphic, in cases in which the telomeric and ancestral copy is more active (Antonell et al. 2005). The two theoretically normofunctional GTF2IRD2 variants have opposite associations with hypertension: 2T+2M that never entails NCF1 deletion is strongly associated with hypertension risk, whereas 1M+3T, linked to NCF1 hemizygosity, is associated with protection for hypertension. On the contrary, the predicted hypomorphic variants are not associated at all with hypertension (P=.33; Fisher’s exact test). These data suggest that NCF1—and not GTF2IRD2—is the major player that determines a different prevalence of hypertension in this population, whereas GTF2IRD2 variants have significant association simply because most rearrangements affect NCF1 and GTF2IRD2 together.
NCF1 encodes a cytosolic subunit of NOX complex, p47phox, which is mutated in the autosomal recessive form of chronic granulomatous disease (MIM 233700). We have demonstrated that NCF1 hemizygosity determines a notable reduction in p47phox protein levels and, consequently, a significant reduction in the ability to generate oxidative stress through decreased activity of the NOX complex. This appears to have a protective effect against hypertension in patients with WBS who are hemizygous for NCF1, who would otherwise be susceptible to hypertension due to vascular narrowing caused by ELN deletion. In fact, there is strong evidence that haploinsufficiency at the ELN locus predisposes to arterial vasculopathy, including hypertension. Animal experimentation has shown that decreased elastin leads to structural changes in vessel walls, with thinner elastic lamellae and an increased number of smooth-muscle cell layers, changes also reported in the arteries of patients with isolated SVAS (Li et al. 1998). These changes in the inner and outer vessel diameter correlate with alterations in transmural pressure. Mice with heterozygous Eln deletions are all stably hypertensive and develop mild cardiac hypertrophy (Faury et al. 2003). These mice have elevated renin levels, and their higher mean arterial pressure can be reduced with angiotensin II inhibitors, which suggests that the renin-angiotensin system plays a significant role in maintaining their high blood pressure.
Compelling evidence has accumulated to support a role for reactive oxygen species (ROS) in various forms of hypertension (Touyz 2005). Chronic infusion of angiotensin II in rats increases vascular NOX-derived ROS preceded by a prominent expression of the p47phox subunit of NOX in the vasculature, macula densa, and distal nephron (Chabrashvili et al. 2002). High intraluminal pressure itself, by activating vascular oxidases, elicits increased superoxide production via activation of the NOX system (Ungvari et al. 2003). Studies performed in Ncf1 knockout mice have revealed that p47phox is one of the major effectors of angiotensin II action. The administration of angiotensin II did not lead to increased superoxide production and elevation of blood pressure in homozygous knockout animals, as it did in wild-type mice (Landmesser et al. 2002). This knockout mouse model somehow reproduces the unique situation in patients with WBS.
We have proven there is dose sensitivity for NCF1 gene expression and resulting effects on NOX activity. We therefore propose that the association found between hypertension and the genotype at NCF1 reflects a significant implication of redox homeostasis in the pathogenesis of hypertension in WBS. Elastin deficiency leads to microscopic vascular narrowing and decreased vascular wall elasticity, regardless of whether it is visible on standard ultrasound or angiogram imaging. This is a predisposing factor to angiotensin II elevation, which may lead to clinical hypertension in only those cases in which the ability to generate higher oxidative stress is normal; that is, when two or more active copies of NCF1 are present (fig. 5). We have also shown that an increase in NCF1 gene-copy number (three copies), present in 13% of patients, does not lead to increased prevalence of hypertension in WBS nor does it determine a greater activation of NOX, despite increased p47phox protein expression. In addition, we found identical frequencies of this genomic variant in a cohort of patients with essential hypertension and in normotensive controls. Therefore, this polymorphic variant does not appear to have a significant influence in the etiology of essential hypertension in the general population. If NCF1 deletion is a protecting factor for hypertension, why does NCF1 duplication not increase the risk? The most likely explanation for this finding is the fact that the NOX complex is heteromultimeric and requires stoichiometric amounts of at least each cytosolic subunit (p40, p47, and p67) (Lapouge et al. 2002), so that an increase in copy number of just one of the subunits may not lead to a significant increase in the oxidase activity. Alternative explanations are that the extra copies of NCF1 might, in fact, be nonfunctional pseudogene(s) due to undetected sequence changes other than the GT insertion/deletion variant and/or that the polymorphic variant might still have some influence, but only when other genetic or environmental factors concur.
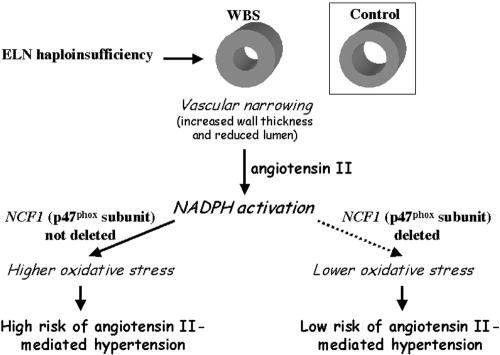
Figure 5.
Proposed pathogenic mechanism of hypertension in WBS. ELN haploinsufficiency in all patients with WBS and a 7q11.23 deletion is known to lead to structural changes in vessel walls mediated by endothelial cell proliferation and smooth-muscle reorganization, resulting in decreased vascular lumen. The resulting increased wall stress would lead to chronic activation of the NOX system via angiotensin II. Quantitative differences in protein levels of the p47phox subunit, depending on deletion subtypes and the resulting NCF1 copy number, determine differences in NADPH enzyme bioavailability. Therefore, patients with a deletion of NCF1 would have a decreased angiotensin II–mediated free-radical production and a decreased risk of hypertension.
Increasing evidence implicates ROS in the pathogenesis of hypertension and its cardiovascular complications (Touyz 2005). By altering the balance in the endothelium between vasoconstrictors and vasodilators such as nitric oxide, ROS contributes to endothelium-dependent contractions and increased vascular resistance. Moreover, the production of peroxynitrite is damaging to the cells through nitrotyrosination of proteins. Antioxidants could restore endothelial function and decrease blood pressure in several models of hypertension, implicating angiotensin II and the redox system (Touyz 2004), but their clinical benefit in essential hypertension in humans remains unconfirmed. In WBS, the results of the present study suggest that antioxidant treatment could have protective effects for hypertension, since the pathogenetic implication of the redox system seems clear. In addition to general antioxidants, anti-NADPH agents (still in clinical trials), the well-known inhibitors of the angiotensin-converting enzyme, and blockers of the angiotensin II receptor could all have a specific indication in patients with WBS. Given that hypertension causes significant morbidity in WBS, contributing to complications such as stroke, cardiac ischemia, and sudden death (Bird et al. 1996; Wollack et al. 1996), the initiation of clinical trials in patients with WBS seems pertinent.
Our results also underscore the relevance and potential success of detailed molecular and clinical characterization of patients with a genomic disorder for obtaining precise correlations that may lead to the determination of causal genes and pathogenic mechanisms. Future detailed evaluation of other highly variable phenotypic features of WBS, such as the complex neurocognitive profile, is warranted.
Acknowledgments
We thank Victoria Campuzano for critical reading, Roser Corominas and Mireia Coma for excellent technical assistance, and Mariano Sentí and Jaume Marrugat for providing samples of hypertensive and control individuals. A.A. was supported by the Departament d'Universitats, Recerca i Societat de la Informarció and Generalitat de Catalunya grant FI02-00790. L.F.M. was supported by the Fondo de Investigación Sanitaria, Spanish Ministry of Health grant FPI-99/9083. This work was supported by grants from the Spanish Ministries of Science and Education (SAF2004-6382) and Health (Networks of Cooperative Research grants C03/07, G03/045, and G03/184), Genome Spain (grant JLI/038), and the Fondation Jerôme Lejeune.
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for WBS, ELN, GTF2I, NCF1, GTF2IRD2 alpha and beta, and chronic granulomatous disease)
References
- Antonell A, De Luis O, Domingo-Roura X, Pérez-Jurado LA (2005) Evolutionary mechanisms shaping the genomic structure of the Williams Beuren syndrome chromosomal region at human 7q11.23. Genome Res 15:1179–1188 10.1101/gr.3944605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayés M, Magano LF, Rivera N, Flores R, Pérez Jurado LA (2003) Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet 73:131–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird LM, Billman GF, Lacro RV, Spicer RL, Jariwala LK, Hoyme HE, Zamora-Salinas R, Morris C, Viskochil D, Frikke MJ, Jones MC (1996) Sudden death in Williams syndrome: report of ten cases. J Pediatr 129:926–931 10.1016/S0022-3476(96)70042-2 [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS (2002) Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39:269–274 10.1161/hy0202.103264 [DOI] [PubMed] [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR (2004) Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A 131:255–264 10.1002/ajmg.a.30400 [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP (2003) Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112:1419–1428 10.1172/JCI200319028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U (1999) Williams syndrome: genes and mechanisms. Hum Mol Genet 8:1947–1954 10.1093/hmg/8.10.1947 [DOI] [PubMed] [Google Scholar]
- Gorlach A, Lee PL, Roesler J, Hopkins PJ, Christensen B, Green ED, Chanock SJ, Curnutte JT (1997) A p47-phox pseudogene carries the most common mutation causing p47-phox-deficient chronic granulomatous disease. J Clin Invest 100:1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg F (1990) Williams syndrome professional symposium. Am J Med Genet Suppl 37:85–88 10.1002/ajmg.1320370615 [DOI] [Google Scholar]
- Hall JG, Froster-Iskenius UG, Allanson JE (eds) (1989) Handbook of physical measurements. Oxford University Press, New York [Google Scholar]
- Heyworth PG, Noack D, Cross AR (2002) Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood 100:1845–1851 10.1182/blood-2002-03-0861 [DOI] [PubMed] [Google Scholar]
- Hirota H, Matsuoka R, Chen XN, Salandanan LS, Lincoln A, Rose FE, Sunahara M, Osawa M, Bellugi U, Korenberg JR (2003) Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on chromosome 7q11.23. Genet Med 5:311–321 [DOI] [PubMed] [Google Scholar]
- Horan MJ (1987) Curves for hypertension in children. Pediatrics 79:1–253797155 [Google Scholar]
- Howald C, Merla G, Digilio MC, Amenta S, Lyle R, Deutsch S, Choudhury U, Bottani A, Antonarakis SE, Fryssira H, Dallapiccola B, Reymond A (2005) Two high-throughput technologies to detect segmental aneuploidies identify new Williams-Beuren syndrome patients with atypical deletions. J Med Genet (http://jmg.bmjjournals.com/cgi/content/abstract/jmg.2005.034009v1) (electronically published July 1, 2005; accessed January 18, 2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365:217–223 [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40:511–515 10.1161/01.HYP.0000032100.23772.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge K, Smith SJ, Groemping Y, Rittinger K (2002) Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase: a central role for p67. J Biol Chem 277:10121–10128 10.1074/jbc.M112065200 [DOI] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT (1998) Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102:1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia R, Pena A, Marrugat J, Sala J, Vida J, Pavesi M, Covas M, Aubo C, Elosúa R, REGICOR Investigators (1998) High prevalence of cardiovascular factors in Girona, Spain, a province with low myocardial infarction incidence. J Epidemiol Community Health 52:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, Sommer A, Moore CA, Hopkin RJ, Spallone PA, Keating MT, Osborne L, Kimberley KW, Stock AD (2003) GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A 123:45–59 10.1002/ajmg.a.20496 [DOI] [PubMed] [Google Scholar]
- Partsch CJ, Dreyer G, Gosch A, Winter M, Schneppenheim R, Wessel A, Pankau R (1999) Longitudinal evaluation of growth, puberty, and bone maturation in children with Williams syndrome. J Pediatr 134:82–89 10.1016/S0022-3476(99)70376-8 [DOI] [PubMed] [Google Scholar]
- Peoples R, Franke Y, Wang Y-K, Pérez-Jurado L, Paperna T, Cisco M, Francke U (2000) A physical map, including a BAC/PAC clone contig, of the Williams-Beuren syndrome–deletion region at 7q11.23. Am J Hum Genet 66:47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Jurado LA (2003) Williams-Beuren syndrome: a model of recurrent genomic mutation. Horm Res Suppl 59:106–113 10.1159/000067836 [DOI] [PubMed] [Google Scholar]
- Pérez Jurado LA, Peoples R, Kaplan P, Hamel BC, Francke U (1996) Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am J Hum Genet 59:781–792 [PMC free article] [PubMed] [Google Scholar]
- Pérez Jurado LA, Wang YK, Peoples R, Coloma A, Cruces J, Francke U (1998) A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of BTK. Hum Mol Genet 7:325–334 10.1093/hmg/7.3.325 [DOI] [PubMed] [Google Scholar]
- Sadler LS, Pober BR, Grandinetti A, Scheiber D, Fekete G, Sharma AN, Urban Z (2001) Differences by sex in cardiovascular disease in Williams syndrome. J Pediatr 139:849–853 10.1067/mpd.2001.118889 [DOI] [PubMed] [Google Scholar]
- Stromme P, Bjornstad PG, Ramstad K (2002) Prevalence estimation of Williams syndrome. J Child Neurol 17:269–271 [DOI] [PubMed] [Google Scholar]
- Tassabehji M (2003) Williams-Beuren syndrome: a challenge for genotype-phenotype correlations. Hum Mol Genet Spec 2 12:R229–R237 10.1093/hmg/ddg299 [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Hammond P, Karmiloff-Smith A, Thompson P, Thorgeirsson SS, Durkin ME, Popescu NC, Hutton T, Metcalfe K, Rucka A, Stewart H, Read AP, Maconochie M, Donnai D (2005) GTF2IRD1 in craniofacial development of humans and mice. Science 310:1184–1187 10.1126/science.1116142 [DOI] [PubMed] [Google Scholar]
- Tipney HJ, Hinsley TA, Brass A, Metcalfe K, Donnai D, Tassabehji M (2004) Isolation and characterisation of GTF2IRD2, a novel fusion gene and member of the TFII-I family of transcription factors, deleted in Williams-Beuren syndrome. Eur J Hum Genet 12:551–560 10.1038/sj.ejhg.5201174 [DOI] [PubMed] [Google Scholar]
- Touyz RM (2004) Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 44:248–252 10.1161/01.HYP.0000138070.47616.9d [DOI] [PubMed] [Google Scholar]
- ——— (2005) Intracellular mechanisms involved in vascular remodeling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol 90:449–455 10.1113/expphysiol.2005.030080 [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A (2003) High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108:1253–1258 10.1161/01.CIR.0000079165.84309.4D [DOI] [PubMed] [Google Scholar]
- Valero MC, de Luis O, Cruces J, Pérez Jurado LA (2000) Fine-scale comparative mapping of the human 7q11.23 region and the orthologous region on mouse chromosome 5G: the low-copy repeats that flank the Williams-Beuren syndrome deletion arose at breakpoint sites of an evolutionary inversion(s). Genomics 69:1–13 10.1006/geno.2000.6312 [DOI] [PubMed] [Google Scholar]
- Wang MS, Schinzel A, Kotzot D, Balmer D, Casey R, Chodirker BN, Gyftodimou J, Petersen MB, Lopez-Rangel E, Robinson WP (1999) Molecular and clinical correlation study of Williams-Beuren syndrome: no evidence of molecular factors in the deletion region or imprinting affecting clinical outcome. Am J Med Genet 86:34–43 [DOI] [PubMed] [Google Scholar]
- Wollack JB, Kaifer M, LaMonte MP, Rothman M (1996) Stroke in Williams syndrome. Stroke 27:143–146 [DOI] [PubMed] [Google Scholar]