Abstract
The micafungin and caspofungin susceptibilities of Candida albicans laboratory and clinical isolates and of Saccharomyces cerevisiae strains stably hyperexpressing fungal ATP-binding cassette (ABC) or major facilitator superfamily (MFS) transporters involved in azole resistance were determined using three separate methods. Yeast strains hyperexpressing individual alleles of ABC transporters or an MFS transporter from C. albicans gave the expected resistance profiles for the azoles fluconazole, itraconazole, and voriconazole. The strains hyperexpressing CDR2 showed slightly decreased susceptibility to caspofungin in agar plate drug resistance assays, as previously reported, but increased susceptibility to micafungin compared with either the strains hyperexpressing CDR1 or the null parent deleted of seven ABC transporters. The strains hyperexpressing CDR1 showed slightly decreased susceptibility to micafungin in these assays. A C. albicans clinical isolate overexpressing both Cdr1p and Cdr2p relative to its azole-sensitive isogenic progenitor acquired resistance to azole drugs and showed reduced susceptibility to caspofungin and slightly increased susceptibility to micafungin in agar plate drug resistance assays. None of the strains showed significant resistance to micafungin or caspofungin in liquid microdilution susceptibility assays. The antifungal activities of micafungin and caspofungin were similar in agarose diffusion assays, although the shape and size of the caspofungin inhibitory zones were affected by medium composition. The assessment of micafungin and caspofungin potency is therefore assay dependent; the differences seen with agar plate drug resistance assays occur over narrow ranges of echinocandin concentrations and are not of clinical significance.
Systemic fungal infection is a problem of increasing clinical significance for the immunocompromised, especially organ transplant recipients and cancer patients. Until recently only three classes of antifungal drugs were available to treat people with these infections: the polyene antibiotics (e.g., amphotericin B), the azole drugs (imidazoles and triazoles), and fluoropyrimidines (flucytosine). The candins, such as caspofungin (CSF) and micafungin (MCF), are members of a novel class of antifungals. They inhibit glucan synthase activity and the synthesis of the essential cell wall component β-1,3-glucan, and they provide new therapeutic options for the treatment of systemic Aspergillus and Candida infections (6). CSF (Cancidas; Merck), an artificial derivative of echinocandin (12), was the first candin to gain FDA approval. MCF (Mycamine; Fujisawa Pharmaceutical Co., now Astellas Pharma Inc.) is a new semisynthetic echinocandin derived from a lead compound obtained from the soil organism Coleophoma empetri (34). Both candins are effective against medically important yeasts and filamentous fungi including Aspergillus fumigatus, Candida albicans, non-C. albicans Candida species, and azole-resistant Candida species (3, 6, 28, 33). Their antifungal spectrum is limited, however, because some clinically important fungal species, including Cryptococcus neoformans, Trichosporon spp., and Fusarium spp., are not susceptible and the zygomycetes (Mucor, Rhizopus, or Absidia) appear innately resistant (10, 35). Despite the ready selection in vitro of candin-resistant Saccharomyces cerevisiae (7) and C. albicans (13) variants with mutations in the catalytic subunit of glucan synthase (27), reports of clinical Candida isolates with acquired candin resistance are rare. Because the candins are not structurally or functionally related to the polyene or azole drugs, cross-resistance to these agents was expected to be infrequent. However, clinical isolates that are resistant to both candins and azoles have been reported for C. albicans (9) and Candida parapsilosis (20). The overexpression of ABC transporters in azole-resistant cells is a concern because they confer resistance to a wide range of structurally unrelated xenobiotics. It was therefore important to determine whether candin susceptibility could be affected by the overexpression of such transporters. Schuetzer-Muehlbauer et al. (30) used MIC determinations and agar plate resistance assays for S. cerevisiae and C. albicans cells overexpressing plasmid-borne copies of C. albicans CDR1 and CDR2 to suggest that Cdr2p confers CSF resistance. In contrast, studies of mutant laboratory strains (5) and clinical isolates (3) concluded that CSF was not a substrate of C. albicans ABC transporters. However, individual efflux pumps that are stably expressed have yet to be fully analyzed for their abilities to pump candins.
We have used a Saccharomyces cerevisiae heterologous membrane protein hyperexpression system to construct a panel of mutant strains that hyperexpress individual transporter alleles cloned from fungal pathogens (19, 23, 25). The panel allows functional analysis of membrane transporters that confer resistance to xenobiotics by using simple drug susceptibility and biochemical assays (21, 36, 37). Here we demonstrate that the functional hyperexpression of single fungal transporters in S. cerevisiae confers detectable candin-specific susceptibility changes in agar plate drug resistance assays. These effects are unlikely to involve the transport activity of known azole efflux pumps or to have clinical significance, because they are not reflected in other assays of drug susceptibility, including those with azole-resistant clinical isolates of C. albicans.
MATERIALS AND METHODS
Yeast strains and culture conditions.
The yeast strains used in this study are listed in Table 1 (see also Table S1 in the supplemental material). S. cerevisiae cells were routinely maintained on CSM-Ura (complete synthetic medium without uracil) agar plates (22). Uridine (50 μg/ml) was added to CSM-Ura for growth of the AD1-8u− host. C. albicans cells were routinely maintained on yeast extract-peptone-dextrose (YPD) agar plates (22).
TABLE 1.
Yeast strains used in this study
| Species and strain | Source | Reference |
|---|---|---|
| Candida albicans | ||
| ATCC 10261 | American Type Culture Collection, Manassas, Va. | |
| TIMM3163 | Clinical isolate, resistant to azoles, from Teikyo University (Tokyo, Japan) | 15 |
| SC5314 | Candida albicans database strain | 11 |
| TL1 | Clinical isolate, sensitive to azoles, obtained from T. White (Seattle, WA) | 17 |
| TL3 | Clinical isolate, resistant to azoles, obtained from T. White (Seattle, WA) | 17 |
| Candida glabrata CBS138 | Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands | |
| Cryptococcus neoformans CDC551 | Serotype A, sexual type α, from Chiba University (Chiba, Japan) |
Plasmid construction and yeast transformation.
KOD DNA polymerase (Toyobo, Osaka, Japan) was used to PCR amplify the open reading frames (ORFs) of fungal ABC transporters or major facilitator superfamily (MFS) transporters (C. albicans CDR1, CDR2, and MDR1, Candida glabrata CDR1, and C. neoformans MDR1) from genomic DNAs of the strains listed in Table 1. The ORFs were directionally cloned into PacI and NotI sites of the pABC3 vector (18). S. cerevisiae AD1-8u− cells (21) were transformed by the lithium acetate method (Alkali-Cation Yeast Transformation kit; Bio 101, Irvine, CA) using a transformation cassette from pABC3 that contained the ORF. Ura+ transformants with the ORF integrated into the PDR5 chromosomal locus were selected on CSM-Ura plates and tested for fluconazole (FLC) resistance on FLC-containing CSM-Ura plates. The cloned genes from Ura+ FlcR transformants were PCR amplified from genomic DNA and their sequences confirmed.
Compounds.
MCF and CSF were synthesized by Fujisawa Pharmaceutical Co., Ltd. (Osaka, Japan). Voriconazole (VRC) was synthesized by the NARD Institute, Ltd. (Hyogo, Japan). Itraconazole (ITC) was purchased from Janssen-Kyowa (Tokyo, Japan). FLC (Diflucan; aqueous solution) was purchased from Pfizer Laboratories Limited (Auckland, New Zealand), and nystatin (NYT) was from Sigma (St. Louis, Mo.). MCF and CSF were dissolved in sterile distilled water, and the other compounds were dissolved in dimethyl sulfoxide.
Preparation of plasma membrane fractions and protein identification.
Plasma membrane fractions were isolated from the S. cerevisiae strains as described by Niimi et al. (22). The expression of ABC proteins (140 to 170 kDa) and MFS protein (60 kDa) was detected as Coomassie blue R250-stained bands with the predicted molecular masses after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in gels containing 8% acrylamide. The identity of each protein band was confirmed by either immunodetection (21) or mass spectrometry tryptic fingerprint analysis (24). The Cdr1p- and Cdr2p-specific polyclonal antibodies were kind gifts from Dominique Sanglard and Martine Raymond, respectively.
Drug susceptibility assays. (i) Agar plate drug resistance assays.
Assays were performed as previously described (30). Cell growth was monitored after incubation at 30°C for 48 h.
(ii) MIC determination by liquid microdilution susceptibility assay and checkerboard drug competition assay.
Antifungal MICs were measured using 96-well microtiter plates and CSM (pH 7.0) buffered with 10 mM morpholineethanesulfonic acid and 20 mM HEPES instead of RPMI (22). MICs for S. cerevisiae and C. albicans strains were determined after 48 h of incubation at 30°C and 24 h of incubation at 35°C, respectively. Each MIC determination was performed in triplicate in at least three independent experiments. Cell growth was monitored at 590 nm using an EL340 Bio Kinetics plate reader (BioTek Instruments). The MICs of MCF and CSF were the concentrations giving >95% growth inhibition (MIC95), while for azoles and NYT the MICs were the concentrations giving >80% and 100% growth inhibition, respectively, compared with the no-drug control. The viability of each strain at and above the MIC was tested by spotting 5-μl samples from microtiter wells onto drug-free YPD agar and incubating at 30°C for 48 h. The minimum fungicidal concentration (MFC) was defined as the lowest concentration of drug that gave no viable colonies on YPD. The checkerboard drug susceptibility assay using candins and the drug efflux pump substrate FLC was performed as previously described (22).
(iii) Drug diffusion assays.
The susceptibilities of yeast strains to candin and azole drugs were compared using diffusion assays in either YPD agar plates with a reduced agar concentration (0.8%) in the overlay, YPD agarose (with 0.6% agarose instead of the agar) plates with 0.4% agarose in the overlay, CSM agar plates with 0.8% agar in the overlay, or CSM agarose (with 0.6% agarose) plates with 0.4% agarose in the overlay. The plates were seeded with 5 × 105 cells in 5 ml of overlay medium. The indicated amount of each drug was applied to individual sterile paper disks and placed on the overlay. Cell growth was monitored after incubation at 30°C for 48 h for S. cerevisiae strains and after 24 h for C. albicans strains.
RESULTS
Overexpression of drug efflux pumps in plasma membrane fractions of S. cerevisiae.
The PDR5 gene from S. cerevisiae, the two alleles (A and B) of CDR1 and of CDR2 from the azole-sensitive C. albicans laboratory strain ATCC 10261, and the A allele of CDR2 from the C. albicans database strain SC5314 were cloned into the PDR5 locus of the hypersensitive S. cerevisiae AD1-8u− host. The construction and analysis of the AD/MDR1 strain (expressing MFS transporter Mdr1p [also called Benrp]) are described elsewhere (25). Yeast strains were also constructed that hyperexpressed the A or B alleles of CDR2 from three clinical isolates obtained from AIDS patients: azole-resistant TIMM3163 (15) and a pair of isogenic strains (the azole-sensitive parent TL1 and the azole-resistant daughter TL3) that were sequentially isolated from another patient during azole treatment (Tables 1 and 2) (17). Allelic variation is absent in the 30-kb region of the SC5314 genome that includes both CDR1 and CDR2 (2, 11).
TABLE 2.
MICs of micafungin and other drugs for S. cerevisiae strains hyperexpressing fungal transporters and for C. albicans strainsa
| Strain | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| MCF | CSF | FLC | ITC | VRC | NYT | |
| S. cerevisiae | ||||||
| AD1-8u− | 0.125 | 1 | 0.5 | 0.031 | 0.063 | 2 |
| AD/CaMDR1 | 0.125 | 1 | 60 | 0.031 | 0.5 | 2 |
| AD/CaCDR1 A | 0.125 | 1 | 200 | >32 | 16 | 2 |
| AD/CaCDR1 B | 0.250 | 1 | 300 | >32 | 16 | 2 |
| AD/CaCDR2 A | 0.125 | 1 | 80 | >32 | 4 | 2 |
| AD/CaCDR2 B | 0.125 | 2 | 160 | >32 | 4 | 2 |
| AD/CgCDR1 | 0.125 | 1 | 300 | >32 | 4 | 4 |
| AD/CnMDR1 | 0.125 | 1 | 5 | >32 | 0.063 | 2 |
| C. albicans | ||||||
| ATCC 10261 | 0.0078 | 0.25 | 0.5 | 0.063 | 0.063 | 8 |
| TIMM3163 | 0.0156 | 0.25 | 32 | 0.5 | 0.5 | 8 |
| TL1 | 0.0078 | 0.5 | 1 | 0.125 | 0.063 | 8 |
| TL3 | 0.0156 | 0.5 | 32 | 0.5 | 0.5 | 8 |
Cells were incubated in CSM (pH 7.0) at 30°C for 48 h for S. cerevisiae and at 35°C for 24 h for C. albicans strains. C. albicans CDR1, CDR2, and MDR1 were from ATCC 10261. Three separate experiments with triplicate determinations each gave identical end points.
Coomassie blue-stained SDS-PAGE profiles of plasma membrane fractions from representative AD/CDR1, AD/CDR2, and AD/PDR5 strains revealed a 170-kDa protein band that was not present in the parental S. cerevisiae host AD1-8u−. The identity of the 170-kDa band was confirmed by Western blot analysis using Cdr1p- and Cdr2p-specific antibodies or by mass spectrometry tryptic fingerprint analysis. The levels of expression of pairs of alleles from either laboratory strains or clinical C. albicans isolates were comparable. The AD/MDR1 strain produced an immunologically identifiable 55-kDa protein band (25). The AD/CDR2 constructs gave consistently lower Pma1p expression than the AD/CDR1 and AD/PDR5 constructs.
Agar plate drug resistance assays.
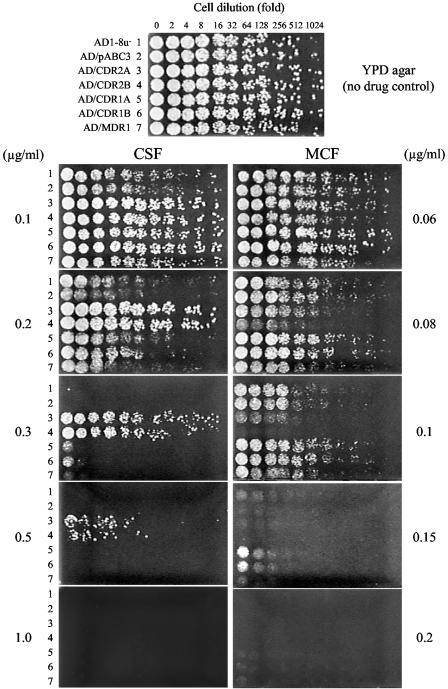
To investigate whether stable hyperexpression of a single genomic copy of CDR2 conferred resistance to MCF and to CSF, as described by Schuetzer-Muehlbauer et al. for plasmid-encoded CDR2 (30), the agar plate drug resistance assay was used for S. cerevisiae strains that hyperexpressed the C. albicans ATCC 10261 CDR2 A or B alleles. The parental strain AD1-8u− and derivative strains transformed with either the empty pABC3 transformation cassette, the CDR1 A or B alleles, or MDR1 (all from ATCC 10261) were used as controls. Each strain was tested using CSF and MCF on YPD plates. Some S. cerevisiae cells hyperexpressing CDR2 survived on YPD agar plates containing ≤0.5 but not 1 μg/ml CSF, while cells of the parental strain (AD1-8u−), AD/pABC3, and those expressing CDR1 or MDR1 were fully susceptible to CSF at 0.3 to 0.5 μg/ml (Fig. 1). In contrast, the AD/CDR2 cells were susceptible to MCF (Fig. 1). The growth of cells expressing either the A or the B allele of CDR2 was inhibited at 0.08 μg/ml MCF, and growth was abolished at 0.1 μg/ml MCF, whereas the parental strain and the other control strains grew at both concentrations.
FIG. 1.
CSF and MCF susceptibilities of S. cerevisiae cells hyperexpressing C. albicans drug efflux pumps in agar plate assays. Alleles were cloned from C. albicans ATCC 10261. Twofold serial dilutions of exponentially growing cells (5 μl) were spotted onto YPD agar medium containing CSF or MCF at the indicated concentrations and incubated at 30°C for 48 h as described in Materials and Methods. Representative results of an experiment carried out three times are shown.
The CSF and MCF susceptibilities of S. cerevisiae strains expressing CDR2 alleles from a variety of azole-resistant and -sensitive C. albicans strains were measured (see Fig. S1 in the supplemental material). The growth of the control strain AD/pABC3 was abolished by CSF at 0.2 μg/ml, but colonies of all the strains hyperexpressing CDR2 survived on YPD agar containing ≤0.6 μg/ml CSF. In contrast, each AD/CDR2 construct was more susceptible to 0.1 μg/ml MCF than the AD/pABC3 control, and no cells survived on agar containing 0.2 μg/ml MCF (see Fig. S1 in the supplemental material).
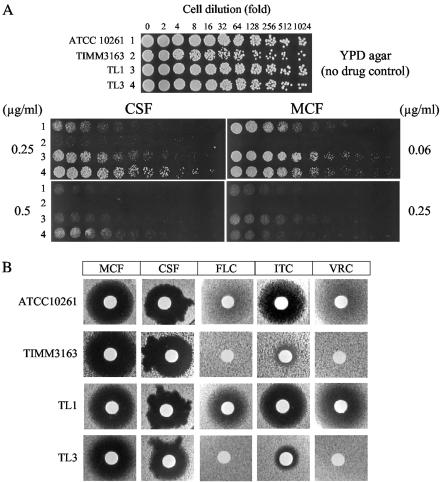
In order to determine whether elevated expression of drug efflux pumps, in particular Cdr2p, is associated with reduced candin susceptibility in C. albicans, we performed agar plate drug resistance assays for candins on YPD agar with the C. albicans strains from which the CDR2 alleles were cloned (Table 1; see also Table S1 in the supplemental material). We previously showed that strains TL3 and TIMM3163 had elevated plasma membrane expression of the Cdr1p and Cdr2p efflux pumps (as determined by Western blot analysis [15] [A. R. Holmes, unpublished results]), and increased energy-dependent efflux of the Cdr substrate R6G, relative to the laboratory strain ATCC 10261 and TL1 (unpublished results). TIMM3163 was highly susceptible to both candins in agar plate drug resistance assays, whereas the other strains survived 0.25 μg/ml CSF and 0.063 μg/ml MCF. There were subtle differences in susceptibility to CSF and MCF between TL1 and TL3. The drug pump-overexpressing daughter strain (TL3) survived 0.5 μg/ml CSF, while the parental strain, TL1, did not. In contrast, TL1 grew slightly better than TL3 at 0.25 μg/ml MCF (Fig. 2A). However, these effects occurred within narrow ranges of candin concentrations. Identical results with agar plate drug resistance assays were obtained in at least three replicate experiments.
FIG. 2.
CSF and MCF susceptibilities of a C. albicans laboratory strain and of clinical isolates from which CDR1 and CDR2 were cloned. (A) The agar plate resistance assay was performed as for Fig. 1. (B) Susceptibilities of C. albicans strains to candins and azoles were determined by a drug diffusion assay on YPD agarose. The following amounts (in micrograms) of drugs were applied to disks: MCF, 2; CSF, 2; FLC, 5; ITC, 0.5; VRC, 0.2.
MICs of azoles and candins for yeast strains hyperexpressing transporters.
The significance of the reduced susceptibilities of strains hyperexpressing CDR1 and CDR2 to MCF and CSF, respectively, and the enhanced susceptibility of the strain hyperexpressing CDR2 to MCF in agar plate drug resistance assays were assessed by determining the liquid MICs of MCF, CSF, and azoles for the panel of S. cerevisiae mutants (see Table S1 in the supplemental material). CSM (pH 7.0) was used for MIC determinations (22) because RPMI medium did not support the growth of the S. cerevisiae strains. The MCF and CSF MICs for the parent strain, AD1-8u−, and for cells hyperexpressing drug efflux pumps were all 0.125 to 0.25 μg/ml and 1 to 2 μg/ml, respectively (Table 2). The MFCs of both candins for all the test strains and the parent strain, AD1-8u−, were at least 8- to 16-fold higher than their MICs. Interstrain differences in MIC profiles at sub-MIC drug concentrations were not detected, and all yeast strains gave the same MIC95 (data not shown). As expected, the strains hyperexpressing ABC pumps had MICs for the three azoles that were significantly higher than those for the parental strain, AD1-8u−, while susceptibilities to NYT were not altered. Hyperexpression of CaMDR1 (MFS pump) conferred resistance to FLC and, to a lesser extent, to VRC. Compared with its orthologs, overexpression of the C. neoformans ABC transporter Mdr1p in S. cerevisiae conferred a different resistance to azole drugs, but this strain was just as susceptible to MFC and CSF as the other strains (Table 2).
The candin MICs were also determined for the C. albicans strains by using CSM (pH 7.0). All the strains had MCF and CSF MICs within the ranges of 0.0078 to 0.0156 μg/ml and 0.25 to 0.5 μg/ml, respectively (Table 2).
Drug diffusion susceptibility assays.
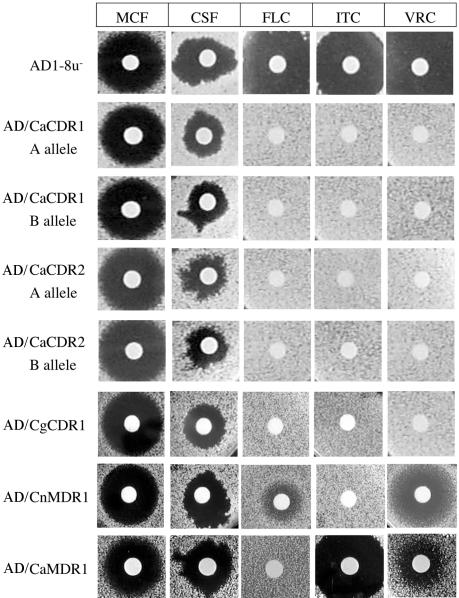
Drug diffusion susceptibility assays were performed using S. cerevisiae strains hyperexpressing C. albicans CDR1 A and B alleles, the CDR2 A and B alleles, CaMDR1, the C. glabrata transporter CgCDR1, and C. neoformans CnMDR1. The sizes of inhibitory zones obtained for CSF on media solidified with agar were significantly smaller than those on media containing agarose (data not shown). Agarose was therefore used instead of agar to enhance the diffusion of the test compounds, especially hydrophobic agents (22). As expected, the parent strain AD1-8u− was sensitive to all three azoles and both candins, while strains hyperexpressing ABC pumps (except CnMDR1) were resistant to all azoles but were sensitive to both candins on both YPD (Fig. 3) and CSM (see Fig. S2 in the supplemental material). There was no difference in sensitivity between cells expressing different C. albicans alleles of CDR1 or CDR2, and all the strains appeared slightly less susceptible to CSF on YPD medium than the parent strain, but the irregular shape of the zones of inhibition made comparison difficult (Fig. 3). This effect was reproducible and medium specific; it was not seen with CSM agarose (see Fig. S2 in the supplemental material). MCF, unlike CSF, gave similar-size circular inhibitory zones independent of medium composition or solidifier. We also performed azole and candin drug diffusion susceptibility assays on YPD agarose for the four C. albicans strains. Both TL3 and TIMM3163 showed significantly higher resistance to the azoles FLC, ITC, and VRC in the agarose drug diffusion assay than strains ATCC 10261 and TL1, but neither of the azole-resistant strains showed modified susceptibility to either MCF or CSF (Fig. 2B). These results were replicated in three independent experiments.
FIG. 3.
Susceptibilities of S. cerevisiae strains hyperexpressing membrane transporters to candins and azoles were determined by a drug diffusion assay on YPD agarose. Amounts of drugs applied per disk were the same as for Fig. 2B except for CSF (1 μg).
Checkerboard drug competition assays.
Competition between the candins and the known drug efflux pump substrate FLC was tested using checkerboard MIC assays. In the strains hyperexpressing C. albicans CDR1, CDR2, or MDR1, no competition was detected between FLC and either CSF or MCF (data not shown).
DISCUSSION
Schuetzer-Muehlbauer et al. (30) used agar plate drug resistance assays of S. cerevisiae and C. albicans strains overexpressing plasmid-borne C. albicans multidrug efflux pumps to conclude that CDR2 conferred hyper-resistance to CSF. We used the same assay to show that the stable, functional, genomic hyperexpression of CaCDR2 alleles obtained from either laboratory strains or clinical isolates conferred on S. cerevisiae reduced susceptibility to CSF but enhanced susceptibility to MCF, in each case over a narrow concentration range of the candin drug. Furthermore, comparable hyperexpression of CaCDR1 alleles conferred on yeast reduced susceptibility to MCF only over an even narrower concentration range. The modified MCF and CSF susceptibilities conferred by hyperexpression of CaCDR1 and CaCDR2 alleles are unlikely to correlate with pump efflux activity, as was seen with the high-level azole resistance conferred by pump overexpression in yeast (23), or to have clinical significance, because these strains gave liquid MICs and drug diffusion inhibitory zones in CSM that were comparable with those of the null parent. Four C. albicans strains were also tested in the three separate susceptibility assays. Two of the strains (TL3 and TIMM3163) were cross resistant to azole drugs and had higher functional expression of Cdr1p and Cdr2p than two azole-sensitive strains (ATCC 10261 and TL1 [15] [A. R. Holmes, unpublished observations]). There were subtle differences between strains TL1 and TL3 in survival on CSF- or MCF-containing agar plates, comparable to the differences seen for the S. cerevisiae strains overexpressing drug pumps (Cdr1p and Cdr2p) and the parent AD1-8u−, but all four strains were equally susceptible to both MCF and CSF in agarose drug diffusion and liquid microdilution susceptibility assays. These observations, which include an isogenic pair of isolates from the same patient (TL1 and TL3 [17]), confirmed that the increased functional expression of Cdr1p and Cdr2p did not give significant candin resistance in C. albicans strains.
Hyperexpression of Cdr2p in the plasma membrane substantially reduced the amount of the essential plasma membrane proton pump expression in membrane fractions, and this effect may cause the reduced growth rates that were observed. Furthermore, Cdr1p and Cdr2p may be phospholipid flippases (31), and energy-independent facilitated diffusion has been implicated in CSF uptake by C. albicans at low drug concentrations (∼1 μg/ml), but it is not known if the latter transport mechanism is essential for CSF activity or if MCF uses the same transporter (26). Although the precise roles of Cdr1p and Cdr2p in modifying slightly the susceptibility of yeast to low concentrations of candin antifungals in the agar plate drug resistance assay have yet to be determined, these effects may be related to a time-dependent (time-kill) effect previously detected for MCF with individual C. albicans strains (8). Thus, although the agar plate drug resistance assay is reproducibly highly sensitive to minor differences in drug susceptibility over narrow windows of drug concentrations, it should be complemented by other in vitro assay methods in evaluating candin efficacy.
The growth of resistant variants within the test population can cause the trailing effect seen with FLC-resistant or -susceptible, dose-dependent C. albicans strains (14). Thus, the MIC at which 80% of growth is inhibited is commonly used as an end point for azole drugs. The MCF and CSF MIC profiles of the S. cerevisiae strains in CSM did not show the trailing effect (data not shown). The MICs of the candins (0.125 to 0.25 μg/ml for MCF and 1 to 2 μg/ml for CSF) were therefore determined at >95% inhibition of cell growth. The surviving colonies in test wells above the MIC accounted for less than 20% of the initial inoculum, and no paradoxical growth effect was observed (32). A subtle difference in the susceptibility to CSF of cells hyperexpressing Cdr1p or Cdr2p was detected in agarose drug diffusion assays using YPD, but not in CSM agarose or in liquid CSM MIC assays. Interpretation of the drug diffusion assay for CSF was complicated by the irregular shape of inhibitory zones on YPD and the effects of medium composition and solidifier on efficacy. In contrast, MCF showed consistent inhibitory activity with all strains tested that was independent of the medium and solidifier. This may be due to the higher water solubility of MCF than of CSF and to the fact that CSF is known to form aggregates in solution at higher concentrations (26). The well diffusion method using Casitone agar medium gives data that agree with those obtained by the NCCLS (now CLSI) M27-A2 method for CSF against Candida spp. (16, 21a). The composition of YPD (yeast extract and peptone) may therefore critically affect susceptibility to CSF in solid and liquid media. MCF gave well-correlated agar diffusion and liquid MIC data (by NCCLS M38-A) for Aspergillus spp. (1, 21b), but MCF activity for Candida spp. using different medium types has yet to be fully assessed. Although NCCLS M27-A2 provides a reference method for testing the activity of candins, the MICs are affected by medium composition (4, 29). The MIC and agarose diffusion data obtained for our yeast constructs correlated well for the azoles but less well for MCF and CSF. A comprehensive set of standardized in vitro susceptibility tests is needed for this new class of antifungals. Resistant variants seen in the inhibitory zones with FLC were not found with either candin, suggesting that development of candin resistance may be infrequent in these S. cerevisiae strains.
We have extended the findings of Schuetzer-Muehlbauer et al. (30) for the agar plate drug resistance assay to strains that stably express single alleles encoding some of the most clinically important drug efflux pumps from C. albicans, C. glabrata, and C. neoformans involved in multidrug resistance. MCF and CSF were also effective against C. albicans clinical isolates functionally overexpressing fungal efflux pumps in a variety of assays under different growth conditions. Furthermore, checkerboard assays showed that neither MCF nor CSF competes with the pump substrate FLC for the Cdr1p, Cdr2p, or Mdr1p efflux pump from C. albicans. We therefore predict that the major drug efflux pumps overexpressed in azole-resistant C. albicans, C. glabrata, or C. neoformans strains are unlikely to cause clinically significant cross-resistance to CSF and MCF.
Supplementary Material
Acknowledgments
We are grateful to Ted White for providing clinical isolates of C. albicans, to Aki Kaneko for technical support in MALDI-TOF analysis, and to Dominique Sanglard and Martine Raymond for providing anti-Cdr1p and anti-Cdr2p antibodies, respectively.
This project was supported in part by the National Institutes of Health (R21DE15075-R.D.C.), the Japan Health Sciences Foundation, and Astellas Pharma Inc.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Arikan, S., P. Yurdakul, and G. Hascelik. 2003. Comparison of two methods and three end points in determination of in vitro activity of micafungin against Aspergillus spp. Antimicrob. Agents Chemother. 47:2640-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud, M. B., M. C. Costanzo, M. S. Skrzypek, G. Binkley, C. Lane, S. R. Miyasato, and G. Sherlock. 2005. The Candida Genome Database (CGD), a community resource for Candida albicans gene and protein information. Nucleic Acids Res. 33:D358-D363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, S. P., T. F. Patterson, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 40:2228-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coste, A. T., M. Karababa, F. Ischer, J. Bille, and D. Sanglard. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning, D. W. 2002. Echinocandins: a new class of antifungal. J. Antimicrob. Chemother. 49:889-891. [DOI] [PubMed] [Google Scholar]
- 7.Douglas, C. M., J. A. Marrinan, W. Li, and M. B. Kurtz. 1994. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-d-glucan synthase. J. Bacteriol. 176:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda, F., K. Otomo, T. Nakai, Y. Morishita, K. Maki, S. Tawara, S. Mutoh, F. Matsumoto, and S. Kuwahara. 2002. In vitro activity of a new lipopeptide antifungal agent, micafungin, against a variety of clinically important fungi. Jpn. J. Chemother. 50:8-19. [Google Scholar]
- 11.Jones, T., N. A. Federspiel, H. Chibana, J. Dungan, S. Kalman, B. B. Magee, G. Newport, Y. R. Thorstenson, N. Agabian, P. T. Magee, R. W. Davis, and S. Scherer. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 101:7329-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kartsonis, N. A., J. Nielsen, and C. M. Douglas. 2003. Caspofungin: the first in a new class of antifungal agents. Drug Resist. Updat. 6:197-218. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz, M. B., G. Abruzzo, A. Flattery, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, M. K., L. E. Williams, D. W. Warnock, and B. A. Arthington-Skaggs. 2004. Drug resistance genes and trailing growth in Candida albicans isolates. J. Antimicrob. Chemother. 53:217-224. [DOI] [PubMed] [Google Scholar]
- 15.Maebashi, K., M. Niimi, M. Kudoh, F. J. Fischer, K. Makimura, K. Niimi, R. J. Piper, K. Uchida, M. Arisawa, R. D. Cannon, and H. Yamaguchi. 2001. Mechanisms of fluconazole resistance in Candida albicans isolates from Japanese AIDS patients. J. Antimicrob. Chemother. 47:527-536. [DOI] [PubMed] [Google Scholar]
- 16.Magaldi, S., S. Mata-Essayag, C. Hartung de Capriles, C. Perez, M. T. Colella, C. Olaizola, and Y. Ontiveros. 2004. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 8:39-45. [DOI] [PubMed] [Google Scholar]
- 17.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk, B. C., R. D. Cannon, K. Nakamura, M. Niimi, K. Niimi, D. R. K. Harding, A. R. Holmes, E. Lamping, A. Goffeau, and A. Decottignies. August 2002. Membrane protein expression system and its application. International patent PCT/NZ02/00163.
- 19.Monk, B. C., K. Niimi, S. Lin, A. Knight, T. B. Kardos, R. D. Cannon, R. Parshot, A. King, D. Lun, and D. R. Harding. 2005. Surface-active fungicidal d-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob. Agents Chemother. 49:57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura, K., M. Niimi, K. Niimi, A. R. Holmes, J. E. Yates, A. Decottignies, B. C. Monk, A. Goffeau, and R. D. Cannon. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts, 2nd ed. Approved standard. NCCLS document M27-A2. NCCLS, Wayne, Pa.
- 21b.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS document M38-A. NCCLS, Wayne, Pa.
- 22.Niimi, K., D. R. Harding, R. Parshot, A. King, D. J. Lun, A. Decottignies, M. Niimi, S. Lin, R. D. Cannon, A. Goffeau, and B. C. Monk. 2004. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a d-octapeptide derivative. Antimicrob. Agents Chemother. 48:1256-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niimi, M. 2004. An efficient system for functional hyperexpression of multidrug efflux pumps in Saccharomyces cerevisiae. Nippon Ishinkin Gakkai Zasshi 45:63-69. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 24.Niimi, M., Y. Nagai, K. Niimi, S. Wada, R. D. Cannon, Y. Uehara, and B. C. Monk. 2002. Identification of two proteins induced by exposure of the pathogenic fungus Candida glabrata to fluconazole. J. Chromatogr. B 782:245-252. [DOI] [PubMed] [Google Scholar]
- 25.Niimi, M., S. Wada, K. Tanabe, A. Kaneko, Y. Takano, T. Umeyama, N. Hanaoka, Y. Uehara, E. Lamping, K. Niimi, S. Tsao, A. R. Holmes, B. C. Monk, and R. D. Cannon. 2005. Functional analysis of fungal drug efflux transporters by heterologous expression in Saccharomyces cerevisiae. Jpn. J. Infect. Dis. 58:1-7. [PubMed] [Google Scholar]
- 26.Paderu, P., S. Park, and D. S. Perlin. 2004. Caspofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob. Agents Chemother. 48:3845-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J. Clin. Microbiol. 42:3117-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuetzer-Muehlbauer, M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 31.Smriti, S. Krishnamurthy, B. L. Dixit, C. M. Gupta, S. Milewski, and R. Prasad. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:303-318. [DOI] [PubMed] [Google Scholar]
- 32.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomishima, M., H. Ohki, A. Yamada, H. Takasugi, K. Maki, S. Tawara, and H. Tanaka. 1999. FK463, a novel water-soluble echinocandin lipopeptide: synthesis and antifungal activity. J. Antibiot. (Tokyo) 52:674-676. [DOI] [PubMed] [Google Scholar]
- 35.Uchida, K., Y. Nishiyama, N. Yokota, and H. Yamaguchi. 2000. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J. Antibiot. (Tokyo) 53:1175-1181. [DOI] [PubMed] [Google Scholar]
- 36.Wada, S., M. Niimi, K. Niimi, A. R. Holmes, B. C. Monk, R. D. Cannon, and Y. Uehara. 2002. Candida glabrata ATP-binding cassette transporters Cdr1p and Pdh1p expressed in a Saccharomyces cerevisiae strain deficient in membrane transporters show phosphorylation-dependent pumping properties. J. Biol. Chem. 277:46809-46821. [DOI] [PubMed] [Google Scholar]
- 37.Wada, S., K. Tanabe, A. Yamazaki, M. Niimi, Y. Uehara, K. Niimi, E. Lamping, R. D. Cannon, and B. C. Monk. 2005. Phosphorylation of Candida glabrata ATP-binding cassette transporter Cdr1p regulates drug efflux activity and ATPase stability. J. Biol. Chem. 280:94-103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.