Abstract
1 The disposition of two angiotensin converting-enzyme inhibitor drugs was studied in normal volunteers. One drug was enalapril maleate (MK-421), which requires in vivo esterolysis to yield active inhibitor (MK-422). The other was a lysine analogue of MK-422 (MK-521), which requires no bioactivation. 2 Absorption of enalapril maleate (10 mg, p.o.) was rapid, with peak serum concentrations of enalapril observed 0.5-1.5 h after administration. Based upon urinary recovery of total drug (enalapril plus MK-422), absorption was at least 61%. Bioactivation appeared to be largely post-absorptive. From the ratio of MK-422 to total drug in urine, the minimum extent of bioactivation was estimated at 0.7. 3 A similar dose of MK-521 was absorbed more slowly, reaching peak serum concentrations 6-8 h following drug administration. Minimum absorption, based upon urinary recovery, was 29%. 4 Serum concentration v time profiles for both drugs were polyphasic and exhibited prolonged terminal phases. 5 Recovery in urine and faeces of administered enalapril maleate (intact and as MK-422) was 94%. Recovery of MK-521 was 97%. These results indicate lack of significant metabolism of these agents, apart from the bioactivation of enalapril.
Full text
PDF
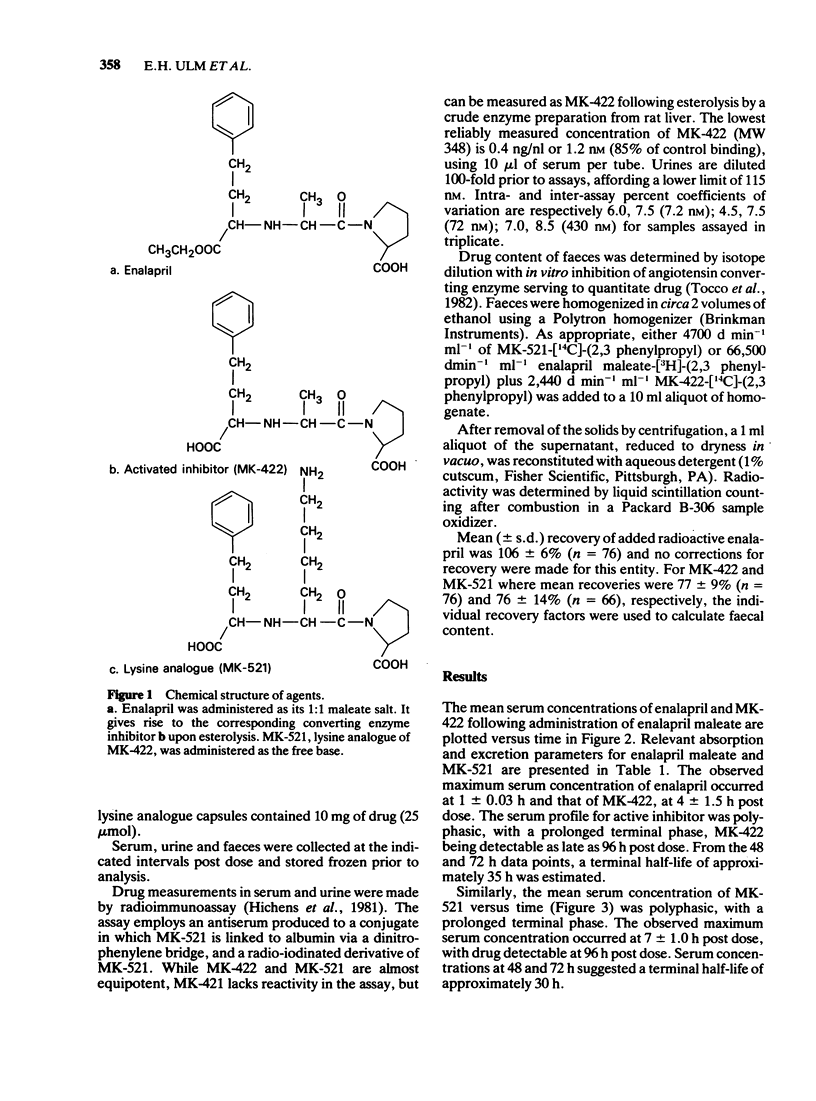
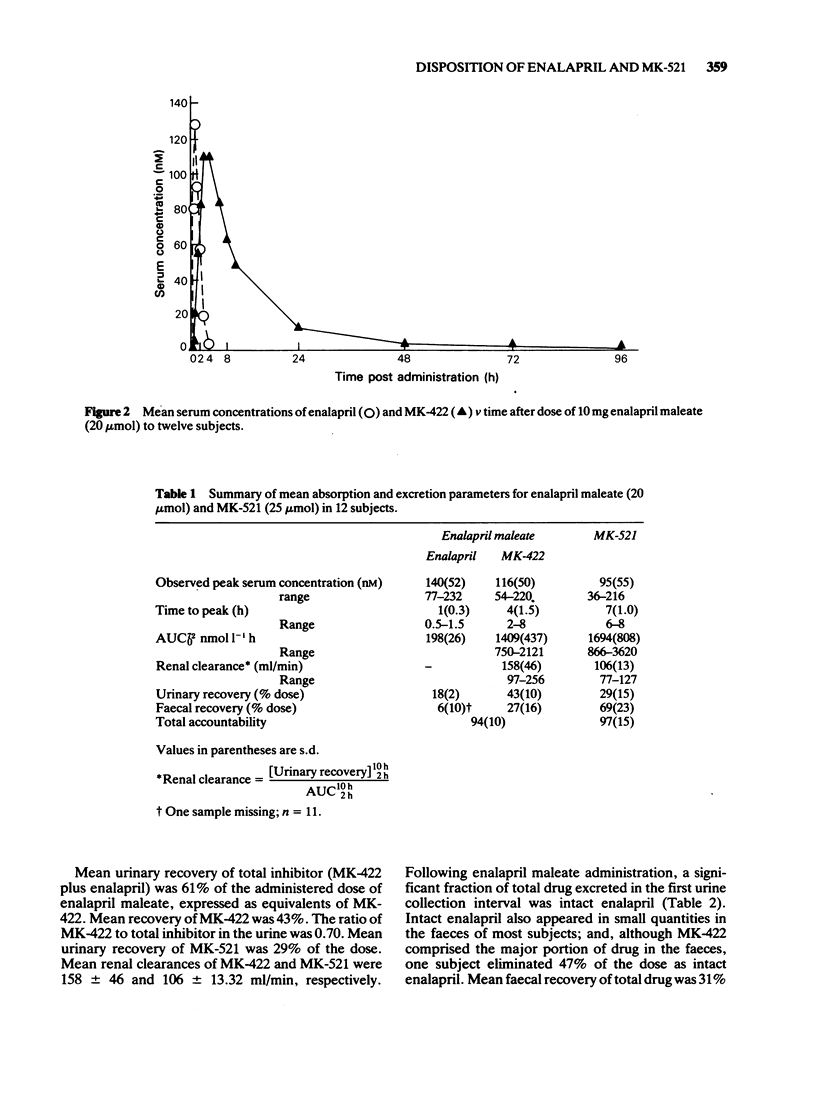
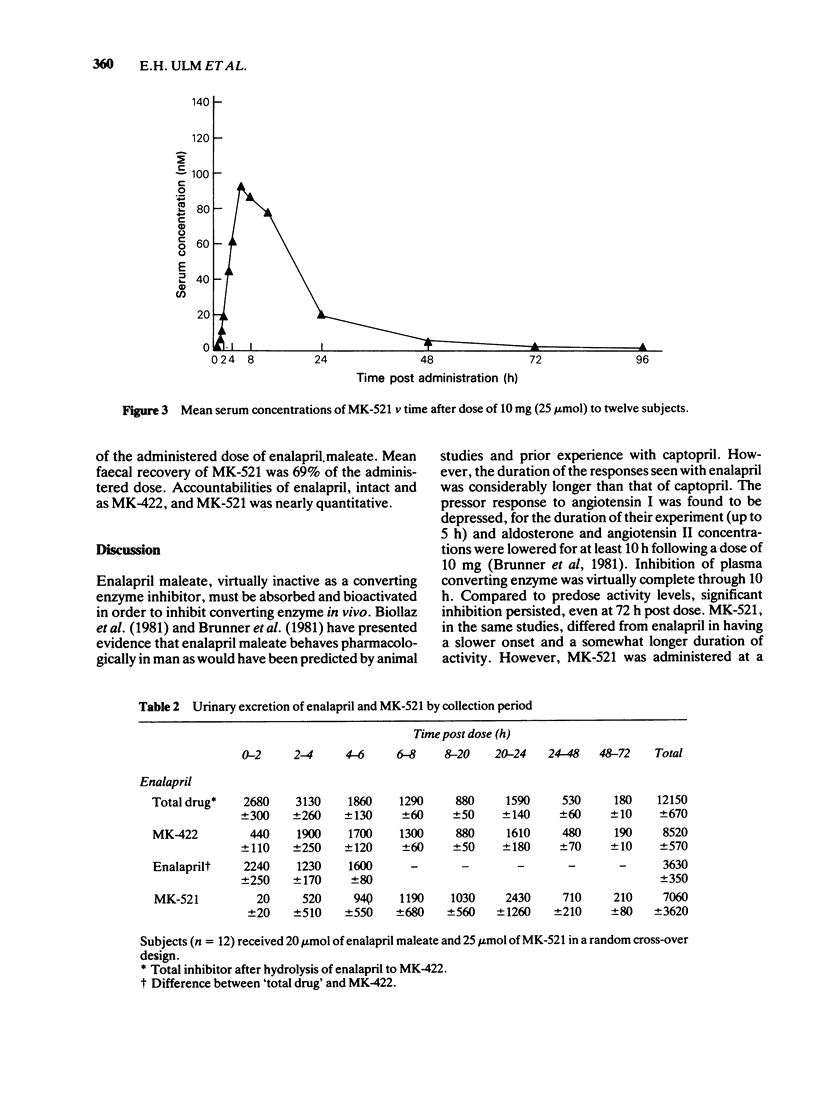


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biollaz J., Burnier M., Turini G. A., Brunner D. B., Porchet M., Gomez H. J., Jones K. H., Ferber F., Abrams W. B., Gavras H. Three new long-acting converting-enzyme inhibitors: relationship between plasma converting-enzyme activity and response to angiotensin I. Clin Pharmacol Ther. 1981 May;29(5):665–670. doi: 10.1038/clpt.1981.92. [DOI] [PubMed] [Google Scholar]
- Biollaz J., Schelling J. L., Jacot Des Combes B., Brunner D. B., Desponds G., Brunner H. R., Ulm E. H., Hichens M., Gomez H. J. Enalapril maleate and a lysine analogue (MK-521) in normal volunteers; relationship between plasma drug levels and the renin angiotensin system. Br J Clin Pharmacol. 1982 Sep;14(3):363–368. doi: 10.1111/j.1365-2125.1982.tb01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D. B., Desponds G., Biollaz J., Keller I., Ferber F., Gavras H., Brunner H. R., Schelling J. L. Effect of a new angiotensin converting enzyme inhibitor MK 421 and its lysine analogue on the components of the renin system in healthy subjects. Br J Clin Pharmacol. 1981 May;11(5):461–467. doi: 10.1111/j.1365-2125.1981.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavras H., Biollaz J., Waeber B., Brunner H. R., Gavras I., Davies R. O. Antihypertensive effect of the new oral angiotensin converting enzyme inhibitor "MK-421". Lancet. 1981 Sep 12;2(8246):543–547. doi: 10.1016/s0140-6736(81)90937-5. [DOI] [PubMed] [Google Scholar]
- Gross D. M., Sweet C. S., Ulm E. H., Backlund E. P., Morris A. A., Weitz D., Bohn D. L., Wenger H. C., Vassil T. C., Stone C. A. Effect of N-[(S)-1-carboxy-3-phenylpropyl]-L-Ala-L-Pro and its ethyl ester (MK-421) on angiotensin converting enzyme in vitro and angiotensin I pressor responses in vivo. J Pharmacol Exp Ther. 1981 Mar;216(3):552–557. [PubMed] [Google Scholar]
- Igic R. P., Gafford J. T., Erdos E. G. Effect of captopril on proteins and peptide hormones. Biochem Pharmacol. 1981 Mar 15;30(6):683–685. doi: 10.1016/0006-2952(81)90150-7. [DOI] [PubMed] [Google Scholar]
- Kripalani K. J., McKinstry D. N., Singhvi S. M., Willard D. A., Vukovich R. A., Migdalof B. H. Disposition of captopril in normal subjects. Clin Pharmacol Ther. 1980 May;27(5):636–641. doi: 10.1038/clpt.1980.90. [DOI] [PubMed] [Google Scholar]
- Patchett A. A., Harris E., Tristram E. W., Wyvratt M. J., Wu M. T., Taub D., Peterson E. R., Ikeler T. J., ten Broeke J., Payne L. G. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980 Nov 20;288(5788):280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- Singhvi S. M., Kripalani K. J., Dean A. V., Keim G. R., Kulesza J. S., Meeker F. S., Jr, Ross J. J., Jr, Shaw J. M., Migdalof B. H. Absorption and bioavailability of captopril in mice and rats after administration by gavage and in the diet. J Pharm Sci. 1981 Aug;70(8):885–888. doi: 10.1002/jps.2600700813. [DOI] [PubMed] [Google Scholar]
- Swanson B. N., Hichens M., Mojaverian P., Ferguson R. K., Vlasses P. H., Dudash M. Angiotensin converting enzyme activity in human serum: relationship to enzyme inhibitor in vivo and in vitro. Res Commun Chem Pathol Pharmacol. 1981 Sep;33(3):525–536. [PubMed] [Google Scholar]
- Sweet C. S., Gross D. M., Arbegast P. T., Gaul S. L., Britt P. M., Ludden C. T., Weitz D., Stone C. A. Antihypertensive activity of N-[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-Ala-L-Pro (MK-421), an orally active converting enzyme inhibitor. J Pharmacol Exp Ther. 1981 Mar;216(3):558–566. [PubMed] [Google Scholar]
- Tocco D. J., deLuna F. A., Duncan A. E., Vassil T. C., Ulm E. H. The physiological disposition and metabolism of enalapril maleate in laboratory animals. Drug Metab Dispos. 1982 Jan-Feb;10(1):15–19. [PubMed] [Google Scholar]


