Abstract
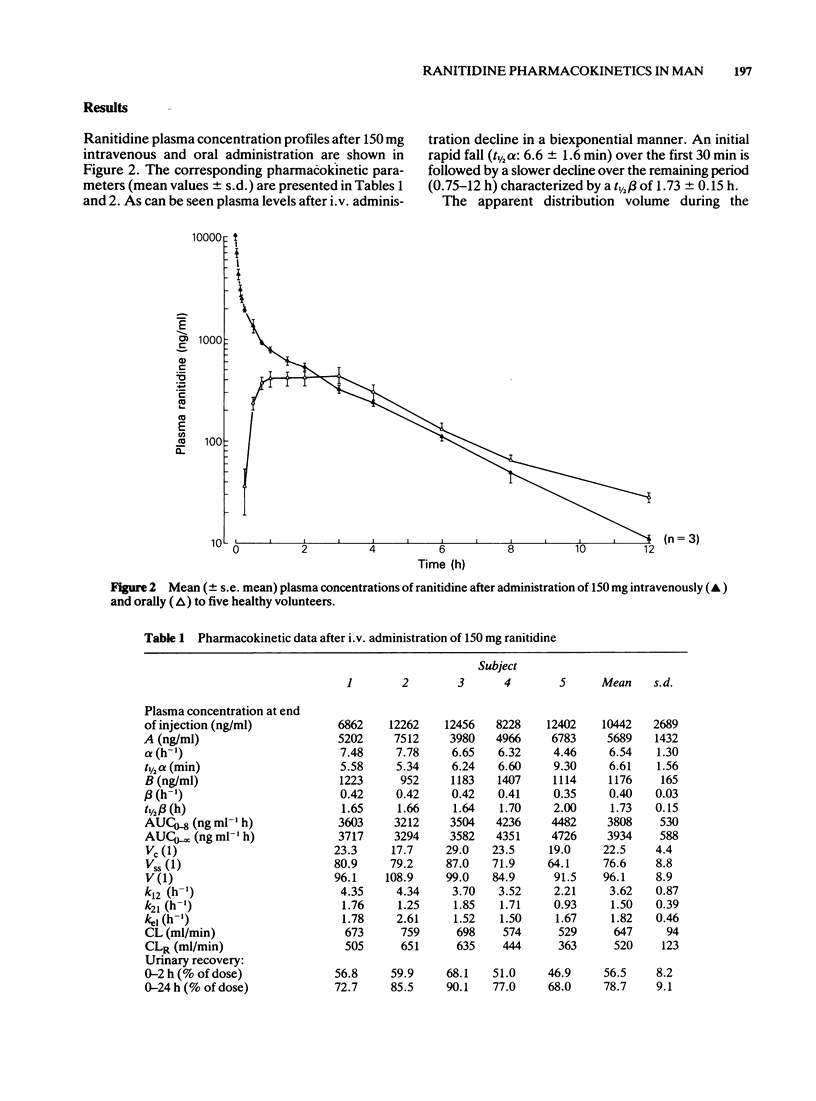
1 Ranitidine single dose pharmacokinetics and absolute bioavailability have been studied in five healthy male volunteers. Following an overnight fast, 150 mg was given intravenously as a bolus injection or orally as a tablet formulation to each subject on separate occasions. 2 Following intravenous administration, plasma levels declined biexponentially. The mean (+/- s.d.) distribution half-life (t 1/2 alpha) was 6.6 +/- 1.6 min; plasma half-life (t 1/2 beta) was 1.7 +/- 0.2 h; the volume of distribution (V) was 96 +/- 9 1; total body clearance (CL) was 647 +/- 94 ml/min and renal clearance (CLR) 520 +/- 123 ml/min. 3 Following oral administration plasma levels showed a bimodal pattern with a first peak at 1.1 +/- 0.4 h and a second peak at 3 +/- 0 h. The absolute availability was 60 +/- 17%. The plasma half-life (t 1/2) of 2.3 +/- 0.4 h was significantly longer (P less than 0.05) after oral than after i.v. administration. 4 Renal excretion of unchanged ranitidine accounted for 79 +/- 9% of the dose after i.v. administration and for 27 +/- 7% after oral administration. 5 Our results suggest a more extensive biotransformation of ranitidine and biliary excretion of metabolites after oral administration while i.v. administration ranitidine is preferentially excreted unchanged in the urine.
Full text
PDF

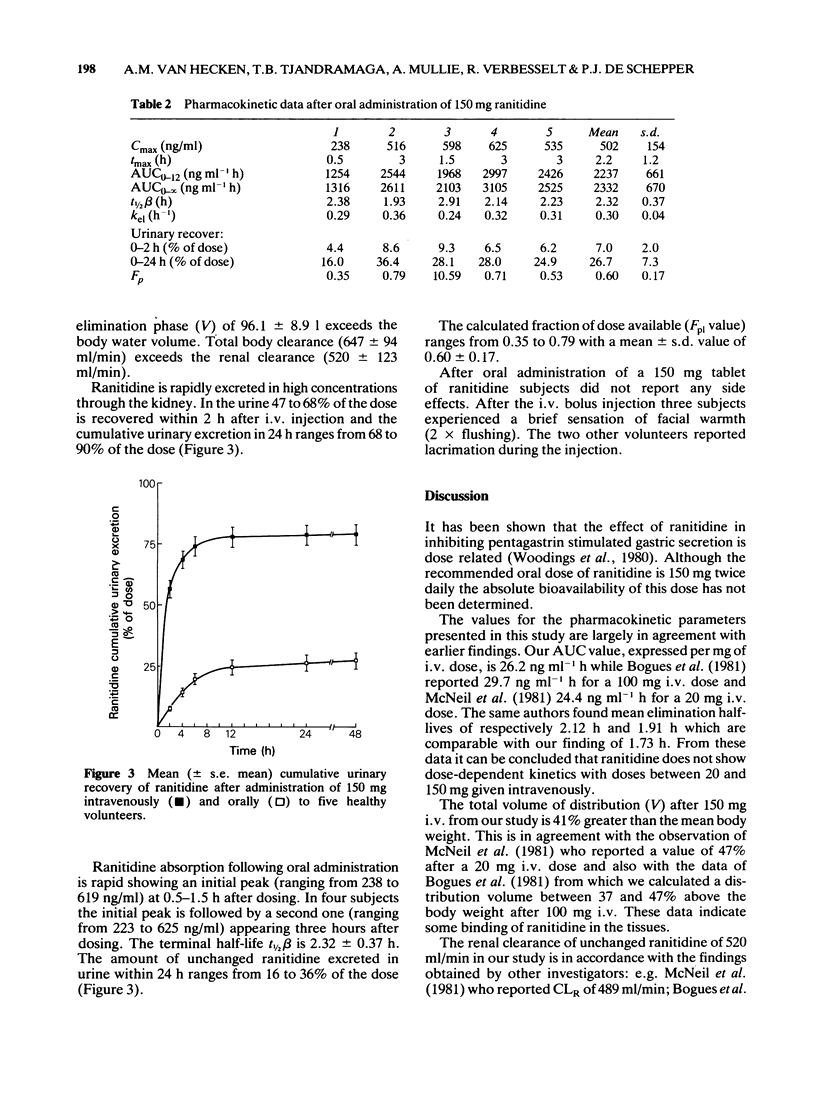


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey P. F., Martin L. E., Owen P. E. Determination of ranitidine and its metabolites in human urine by reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr. 1981 Sep 11;225(1):161–168. doi: 10.1016/s0378-4347(00)80255-8. [DOI] [PubMed] [Google Scholar]
- McNeil J. J., Mihaly G. W., Anderson A., Marshall A. W., Smallwood R. A., Louis W. J. Pharmacokinetics of the H2- receptor antagonist ranitidine in man. Br J Clin Pharmacol. 1981 Sep;12(3):411–415. doi: 10.1111/j.1365-2125.1981.tb01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden N. R., Richards D. A., Saunders J. H., Wormsley K. G. Pharmacologically effective plasma concentrations of ranitidine. Lancet. 1979 Jul 28;2(8135):199–200. doi: 10.1016/s0140-6736(79)91466-1. [DOI] [PubMed] [Google Scholar]
- Woodings E. P., Dixon G. T., Harrison C., Carey P., Richards D. A. Ranitidine--a new H2-receptor antagonist. Gut. 1980 Mar;21(3):187–191. doi: 10.1136/gut.21.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


