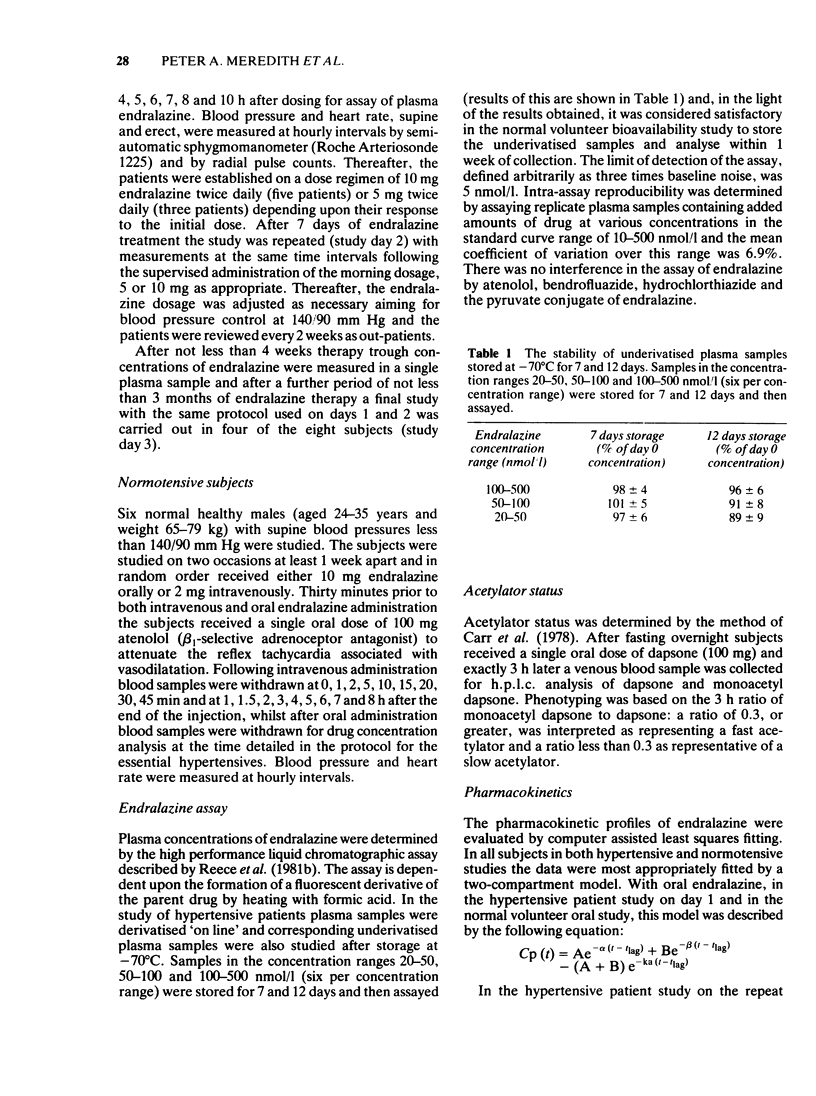
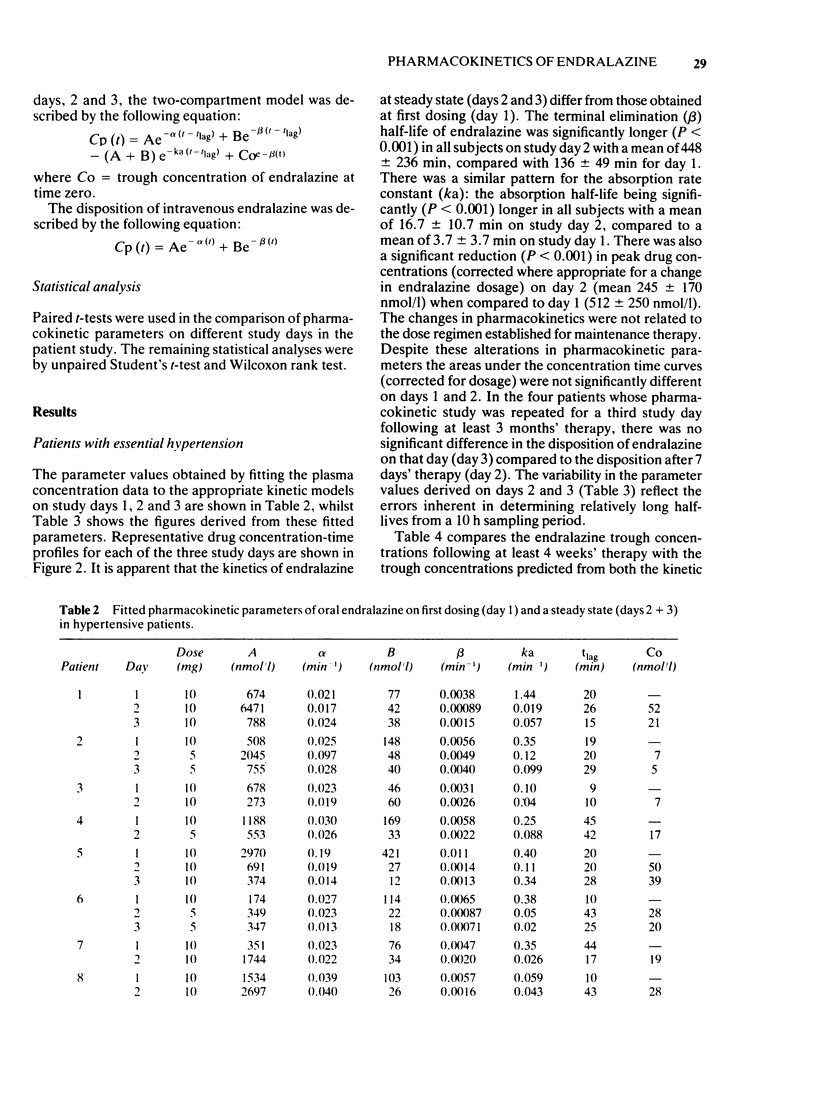
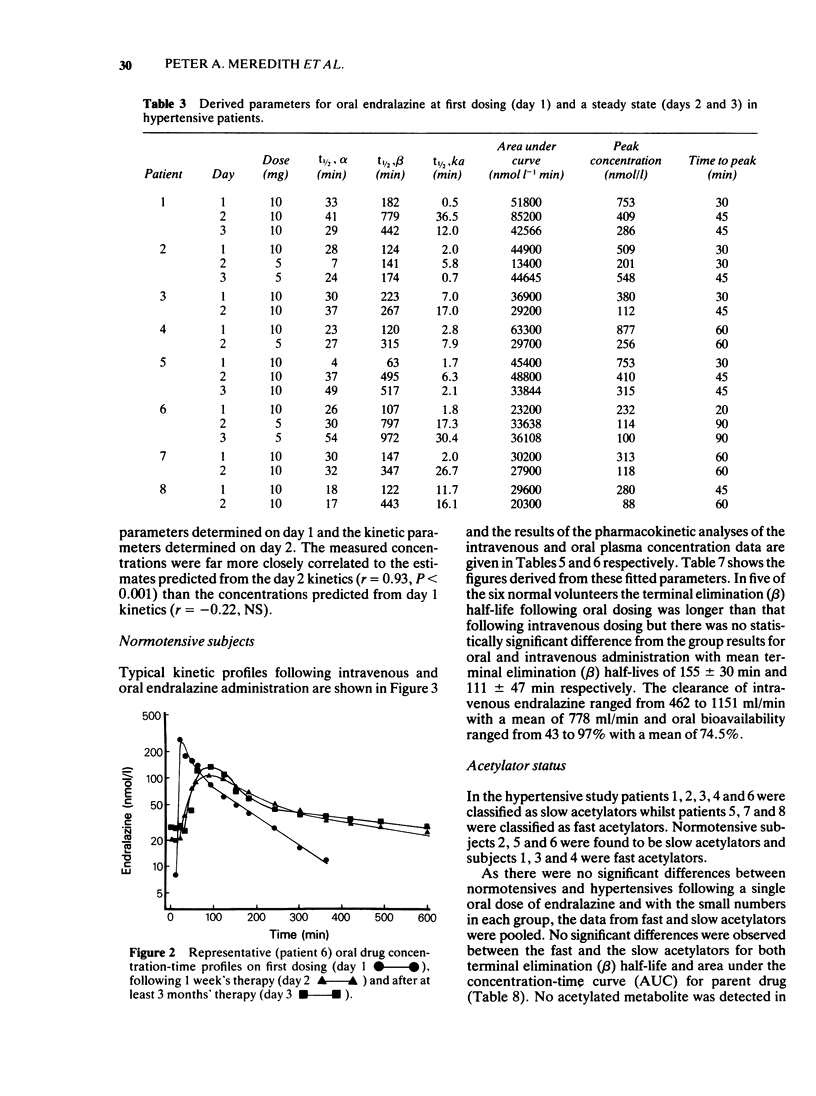
Abstract
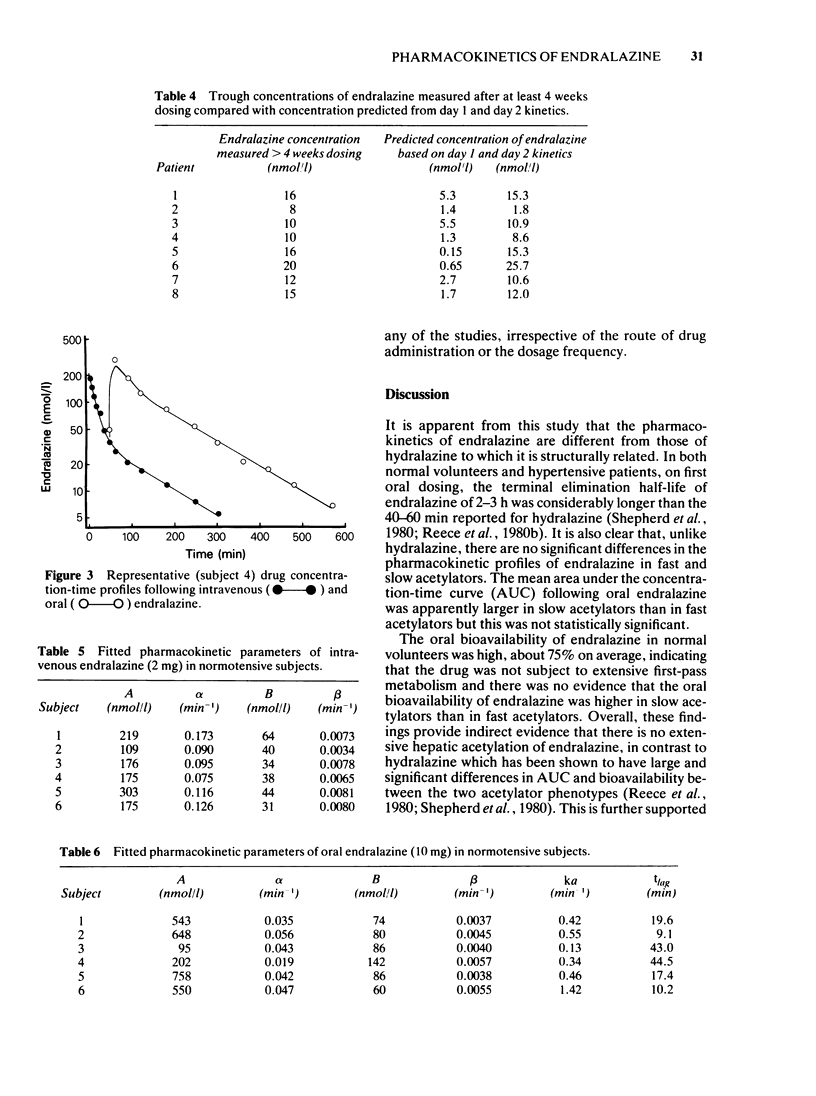
The direct-acting vasodilator, endralazine, in combination with a beta-adrenoceptor blocker significantly reduced the blood pressures of normotensive volunteers and of patients with essential hypertension. The mean terminal elimination half-life for endralazine of 136 min in hypertensive patients did not differ significantly from the 155 min in normotensive subjects. In normal subjects the mean oral bioavailability for endralazine was 75% and the mean clearance was 780 ml/min. There were no significant pharmacokinetic differences between fast and slow acetylators. The administration of endralazine to steady state in the hypertensive patients was associated with an increase in terminal elimination half-life and a decrease in the rate of absorption. However, there was no accumulation of endralazine with chronic dosing.
Full text
PDF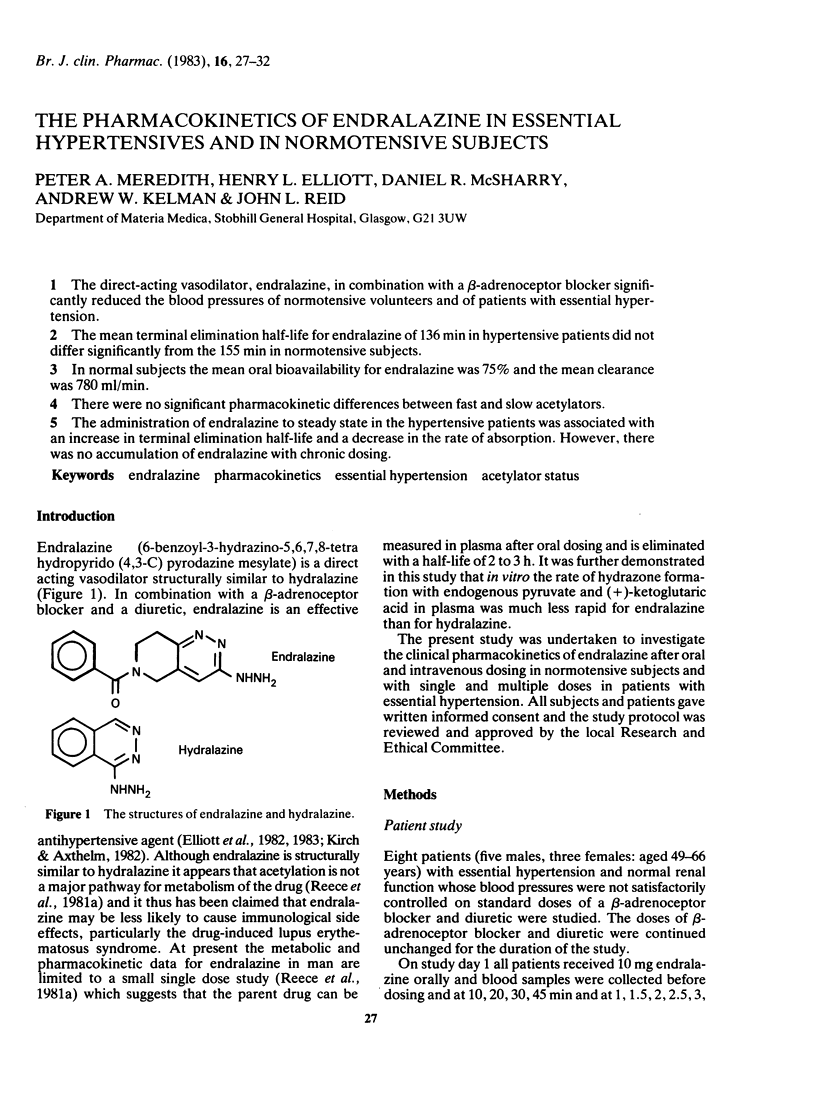
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr K., Oates J. A., Nies A. S., Woosley R. L. Simultaneous analysis of dapsone and monoacetyldapsone employing high performance liquid chromatography: a rapid method for determination of acetylator phenotype. Br J Clin Pharmacol. 1978 Nov;6(5):421–427. doi: 10.1111/j.1365-2125.1978.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott H. L., McLean K., Sumner D. J., Donnelly R. J., Reid J. L. Clinical evaluation of endralazine (BO22708), a new vasodilator, in essential hypertension. Clin Exp Hypertens A. 1982;4(8):1409–1418. doi: 10.3109/10641968209060798. [DOI] [PubMed] [Google Scholar]
- Kirch W., Axthelm T. Endralazine, a new peripheral vasodilator--a randomized cross-over trial against dihydralazine. J Cardiovasc Pharmacol. 1982 Jul-Aug;4(4):562–566. doi: 10.1097/00005344-198207000-00006. [DOI] [PubMed] [Google Scholar]
- Ludden T. M., McNay J. L., Jr, Shepherd A. M., Lin M. S. Clinical pharmacokinetics of hydralazine. Clin Pharmacokinet. 1982 May-Jun;7(3):185–205. doi: 10.2165/00003088-198207030-00001. [DOI] [PubMed] [Google Scholar]
- Reece P. A., Cozamanis I., Zacest R. Kinetics of hydralazine and its main metabolites in slow and fast acetylators. Clin Pharmacol Ther. 1980 Dec;28(6):769–778. doi: 10.1038/clpt.1980.234. [DOI] [PubMed] [Google Scholar]
- Reece P. A., Cozamanis I., Zacest R. Sensitive high-performance liquid chromatographic assay for endralazine and two of its metabolites in human plasma. J Chromatogr. 1981 Sep 11;225(1):151–160. doi: 10.1016/s0378-4347(00)80254-6. [DOI] [PubMed] [Google Scholar]
- Shepherd A. M., Ludden T. M., McNay J. L., Lin M. S. Hydralazine kinetics after single and repeated oral doses. Clin Pharmacol Ther. 1980 Dec;28(6):804–811. doi: 10.1038/clpt.1980.238. [DOI] [PubMed] [Google Scholar]


