Abstract
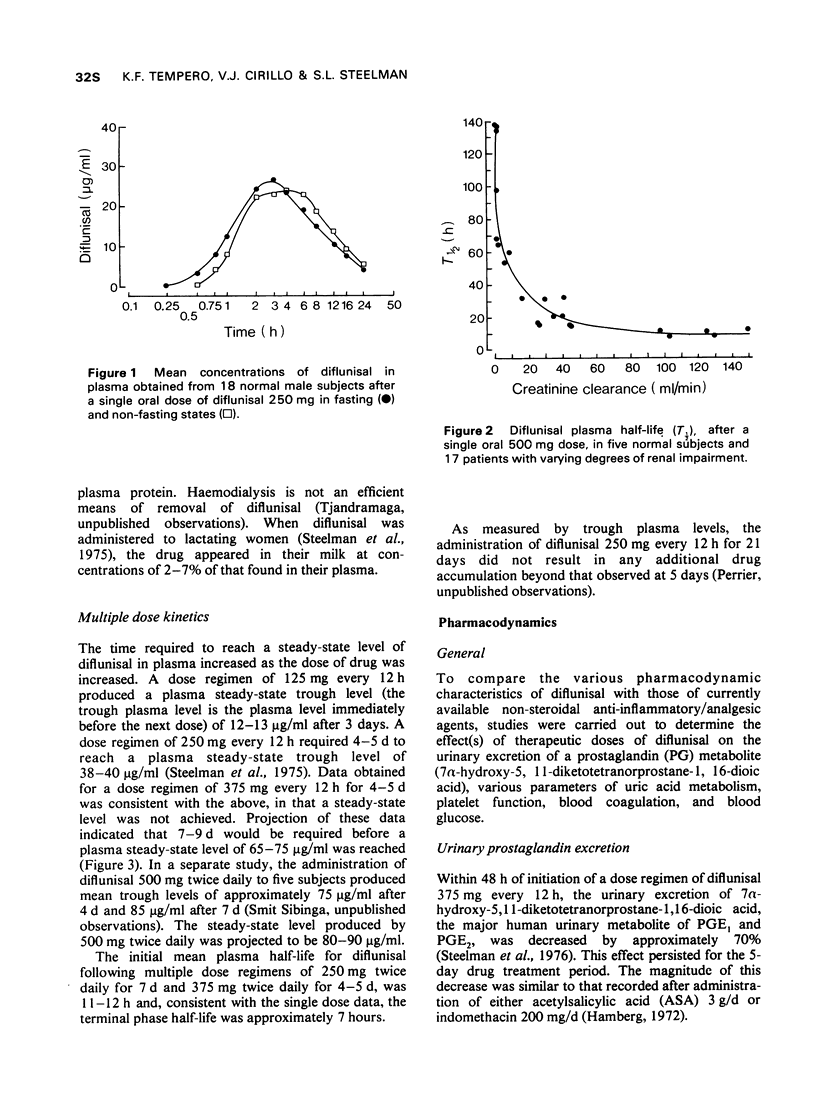
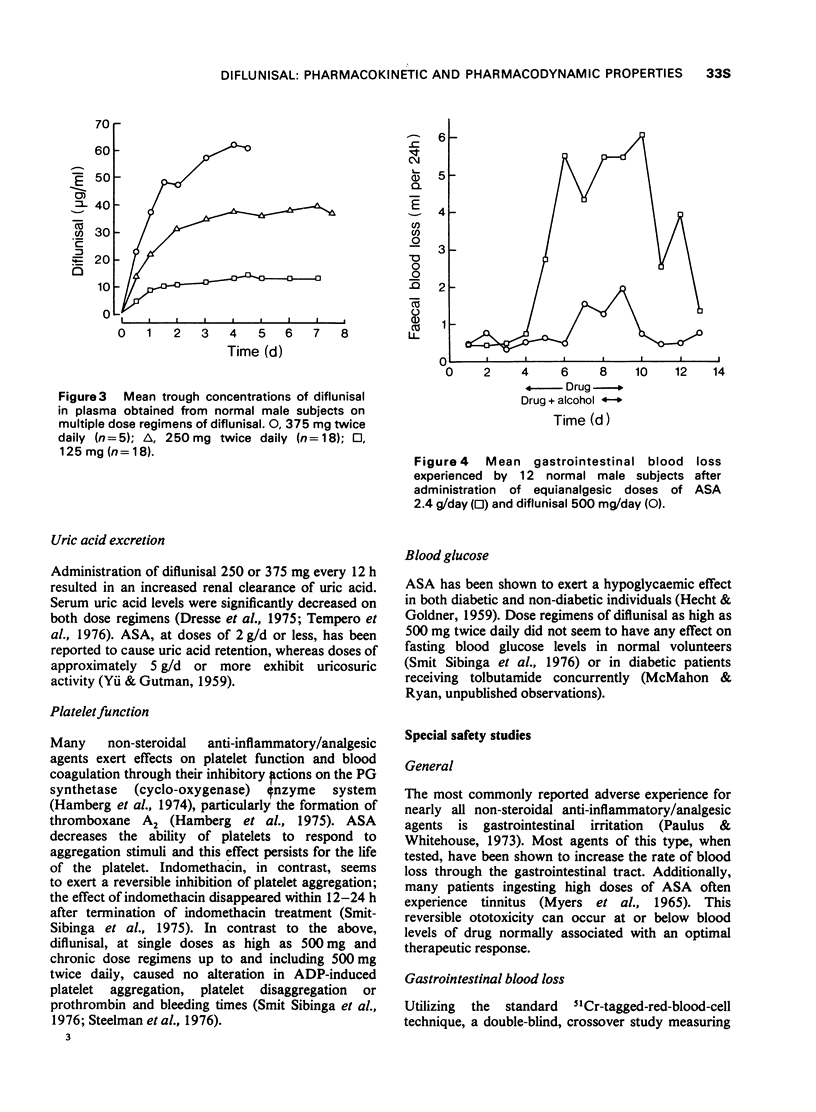
1 In the fasting state, peak plasma levels of diflunisal were achieved within 2 hours. The drug was not metabolized and almost totally excreted in the urine as unchanged or conjugated drug. The terminal plasma half-life was approximately 8 hours. These results support a twice daily dose regimen.
2 During multiple dose administration the time required to achieve steady-state plasma levels varied with the dose. A dose regimen of 125 mg twice daily required 2-3 d, whereas a regimen of 500 mg twice daily required 7-9 d to reach a steady-state plasma level.
3 Clinically effective doses of diflunisal decreased the urinary excretion of the major prostaglandin E metabolite, 7α-hydroxy-5,11-diketotetranorprostane-1,16-dioic acid, and exhibited significant uricosuric activity. These same doses did not seem to cause tinnitus, nor did they significantly alter gastrointestinal blood loss, affect blood glucose, bleeding time, or platelet function.
4 Clinically significant drug interactions may be anticipated during concomitant administration with at least one oral anticoagulant (acenocoumarol), but probably not anticipated during the coadministration of oral antidiabetic agents, thiazide diuretics, and non-steroidal anti-inflammatory/analgesic agents.
5 Clinical and laboratory data accumulated during these studies indicated that diflunisal was well tolerated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HECHT A., GOLDNER M. G. Reappraisal of the hypoglycemic action of acetylsalicylate. Metabolism. 1959 Jul 1;8(4 Pt 1):418–428. [PubMed] [Google Scholar]
- Hamberg M. Inhibition of prostaglandin synthesis in man. Biochem Biophys Res Commun. 1972 Nov 1;49(3):720–726. doi: 10.1016/0006-291x(72)90470-6. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS E. N., BERNSTEIN J. M., FOSTIROPOLOUS G. SALICYLATE OTOTOXICITY: A CLINICAL STUDY. N Engl J Med. 1965 Sep 9;273:587–590. doi: 10.1056/NEJM196509092731104. [DOI] [PubMed] [Google Scholar]
- McLeod P. J., Ogilvie R. I., Ruedy J. Effects of large and small doses of hydrochlorothiazide in hypertensive patients. Clin Pharmacol Ther. 1970 Sep-Oct;11(5):733–739. doi: 10.1002/cpt1970115733. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Whitehouse M. W. Nonsteroid anti-inflammatory agents. Annu Rev Pharmacol. 1973;13:107–125. doi: 10.1146/annurev.pa.13.040173.000543. [DOI] [PubMed] [Google Scholar]
- Tocco D. J., Breault G. O., Zacchei A. G., Steelman S. L., Perrier C. V. Physiological disposition and metabolism of 5-(2',4'-difluorophenyl)salicyclic acid, a new salicylate. Drug Metab Dispos. 1975 Nov-Dec;3(6):453–466. [PubMed] [Google Scholar]
- YU T. F., GUTMAN A. B. Study of the paradoxical effects of salicylate in low, intermediate and high dosage on the renal mechanisms for excretion of urate in man. J Clin Invest. 1959 Aug;38(8):1298–1315. doi: 10.1172/JCI103905. [DOI] [PMC free article] [PubMed] [Google Scholar]


