Abstract
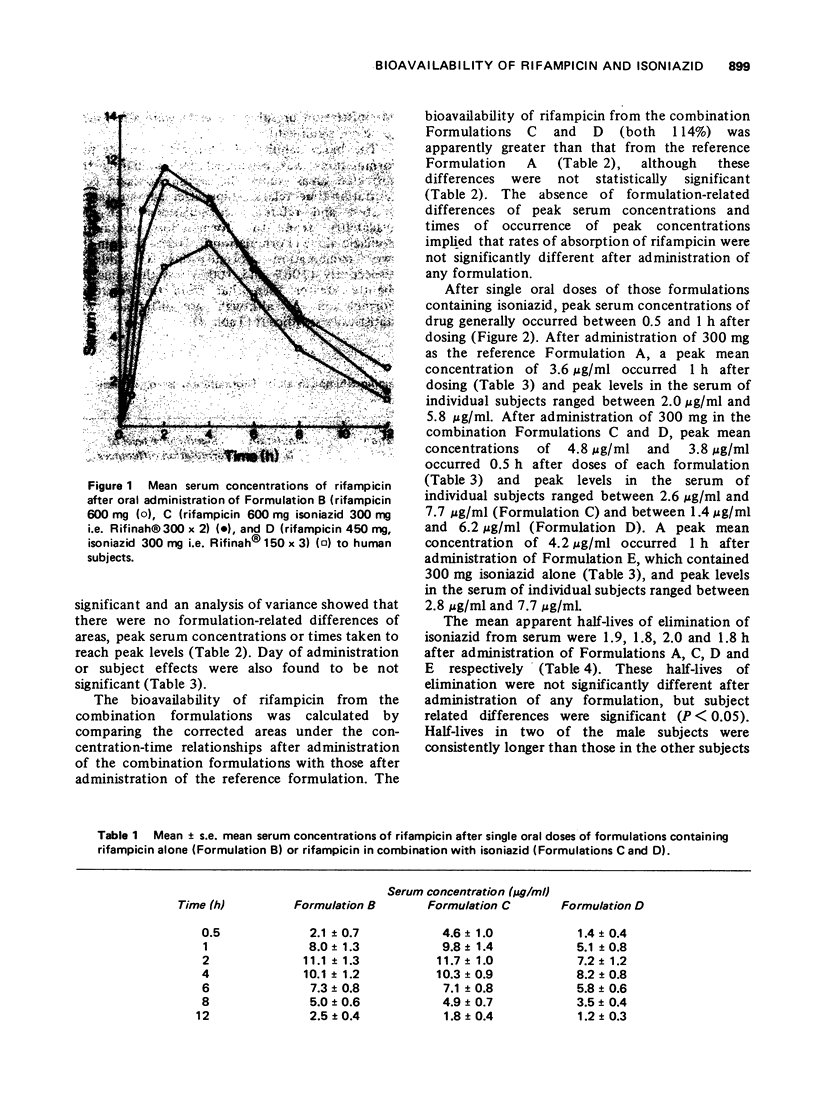
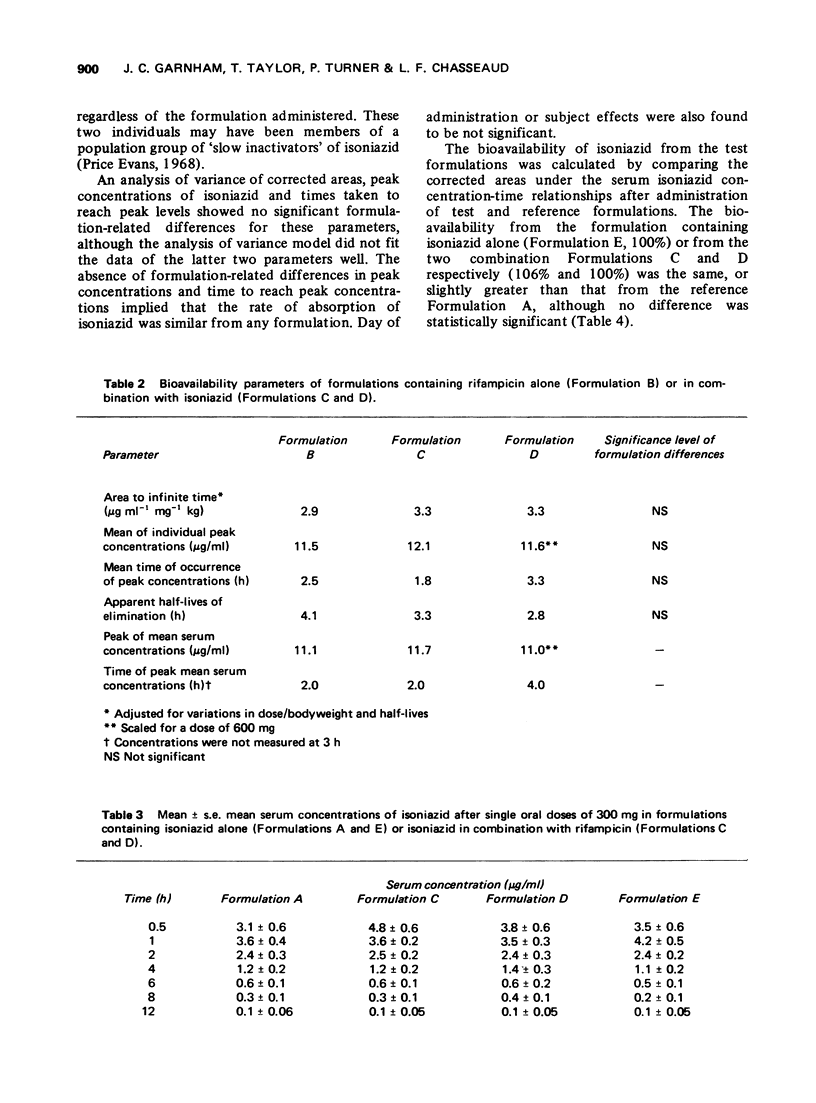
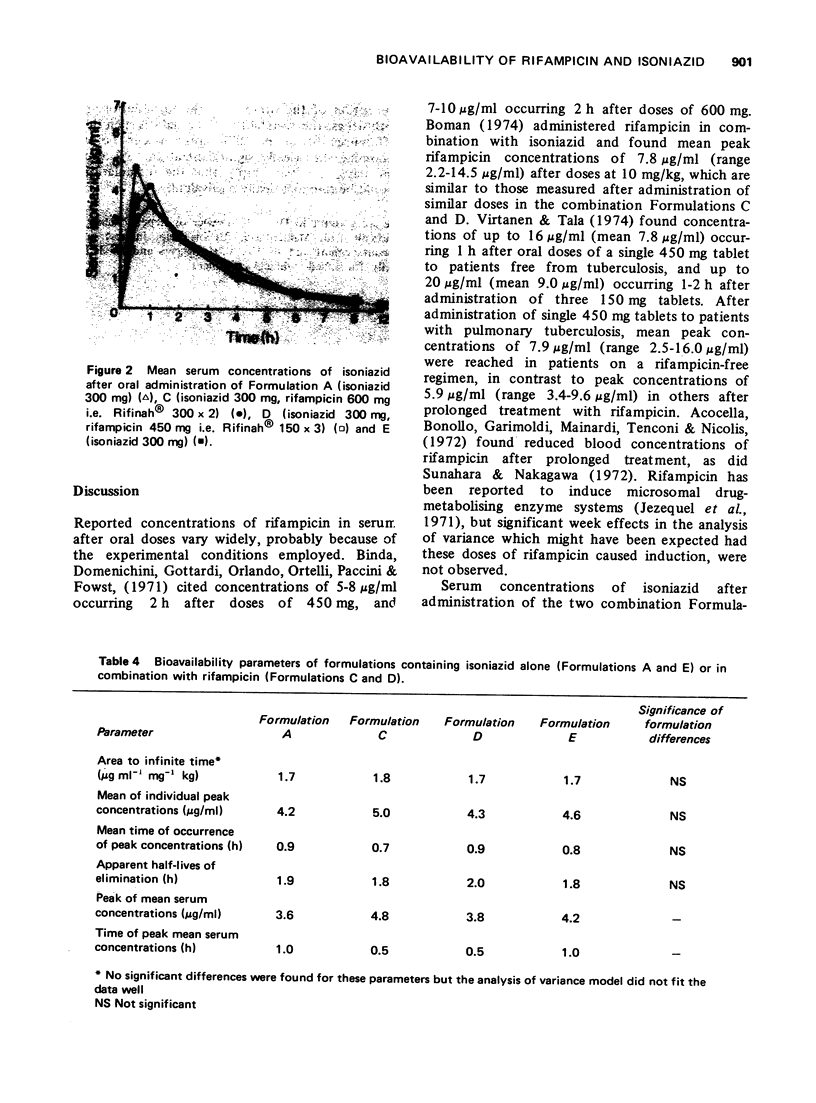
The bioavailability of rifampicin and isoniazid from formulations containing these drugs in combination has been compared to that from formulations containing either drug alone. No formulation-related differences in either rates or extent of bioavailability were found after administration of each formulation. Mean peak serum concentrations of rifampicin (8.2-11.7 mug/ml) occurring 2 to 4 h after doses of 600 mg, and isoniazid (3.6-4.8 mug/ml) occurring 0.5 to 1 h after doses of 300 mg, were similar to those reported in the literature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acocella G., Bonollo L., Garimoldi M., Mainardi M., Tenconi L. T., Nicolis F. B. Kinetics of rifampicin and isoniazid administered alone and in combination to normal subjects and patients with liver disease. Gut. 1972 Jan;13(1):47–53. doi: 10.1136/gut.13.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda G., Domenichini E., Gottardi A., Orlandi B., Ortelli E., Pacini B., Fowst G. Rifampicin, a general review. Arzneimittelforschung. 1971 Dec;21(12):1907–1977. [PubMed] [Google Scholar]
- Boman G., Lundgren P., Stjernström G. Mechanism of the inhibitory effect of PAS granules on the absorption of rifampicin: adsorption of rifampicin by an excipient, bentonite. Eur J Clin Pharmacol. 1975 Jun 13;8(5):293–299. doi: 10.1007/BF00562653. [DOI] [PubMed] [Google Scholar]
- Boman G. Serum concentration and half-life of rifampicin after simultaneous oral administration of aminosalicylic acid or isoniazid. Eur J Clin Pharmacol. 1974;7(3):217–225. doi: 10.1007/BF00560384. [DOI] [PubMed] [Google Scholar]
- CAMPAGNA F. A., CURETON G., MIRIGIAN R. A., NELSON E. Inactive prednisone tablets U.S.P. XVI. J Pharm Sci. 1963 Jun;52:605–606. doi: 10.1002/jps.2600520626. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Lasagna L. How reliable are enteric-coated aspirin preparations? Clin Pharmacol Ther. 1965 Sep-Oct;6(5):568–574. doi: 10.1002/cpt196565568. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Genetic variations in the acetylation of isoniazid and other drugs. Ann N Y Acad Sci. 1968 Jul 31;151(2):723–733. doi: 10.1111/j.1749-6632.1968.tb48255.x. [DOI] [PubMed] [Google Scholar]
- Jezequel A. M., Orlandi F., Tenconi L. T. Changes of the smooth endoplasmic reticulum induced by rifampicin in human and guinea-pig hepatocytes. Gut. 1971 Dec;12(12):984–987. doi: 10.1136/gut.12.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutt H., Brennan R., Dehejia H., Verebely K. Diphenylhydantoin intoxication. A complication of isoniazid therapy. Am Rev Respir Dis. 1970 Mar;101(3):377–384. doi: 10.1164/arrd.1970.101.3.377. [DOI] [PubMed] [Google Scholar]
- LEVY G. EFFECT OF DOSAGE FORM PROPERTIES ON THERAPEUTIC EFFICACY OF TOLBUTAMIDE TABLETS. Can Med Assoc J. 1964 Apr 18;90:978–979. [PMC free article] [PubMed] [Google Scholar]
- Nitti V., Ninni A., Meola G., Iuliano A., Curci G. Comparative investigations of the enzyme-inducing activity of rifampicin and barbiturates in man. Chemotherapy. 1973 Oct;19(4):206–210. doi: 10.1159/000221456. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. A clinical comparison of two preparations of calciferol. Australas Ann Med. 1968 Feb;17(1):56–62. doi: 10.1111/imj.1968.17.1.56. [DOI] [PubMed] [Google Scholar]
- Sunahara S., Nakagawa H. Metabolic study and controlled clinical trials of rifampin. Chest. 1972 Jun;61(6):526–532. doi: 10.1378/chest.61.6_supplement.526. [DOI] [PubMed] [Google Scholar]
- Tyrer J. H., Eadie M. J., Sutherland J. M., Hooper W. D. Outbreak of anticonvulsant intoxication in an Australian city. Br Med J. 1970 Oct 31;4(5730):271–273. doi: 10.1136/bmj.4.5730.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley A. B. The generic inequivalence of drugs. JAMA. 1968 Nov 18;206(8):1745–1748. [PubMed] [Google Scholar]
- Virtanen S., Tala E. Serum concentration of rifampin after oral administration. Clin Pharmacol Ther. 1974 Nov;16(5 Pt 1):817–820. doi: 10.1002/cpt1974165part1817. [DOI] [PubMed] [Google Scholar]
- Wagner J. G. Method of estimating relative absorption of a drug in a series of clinical studies in which blood levels are measured after single and/or multiple doses. J Pharm Sci. 1967 May;56(5):652–653. doi: 10.1002/jps.2600560527. [DOI] [PubMed] [Google Scholar]


