Abstract
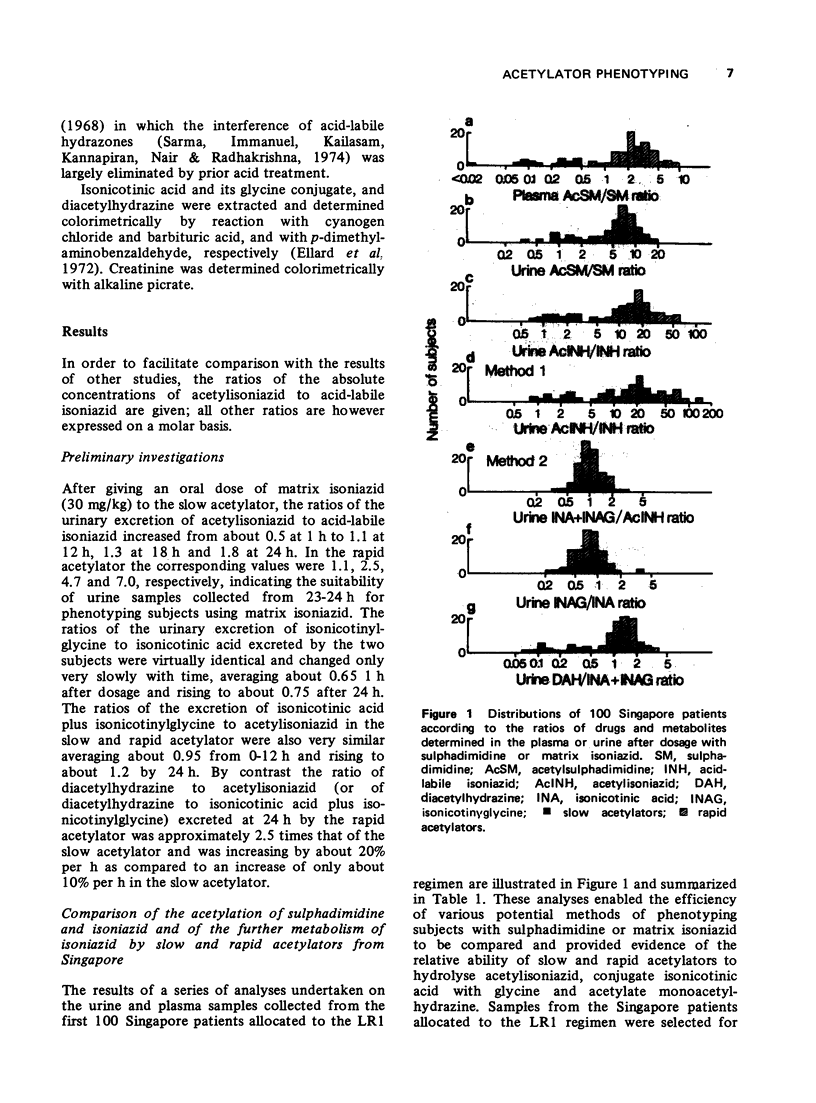
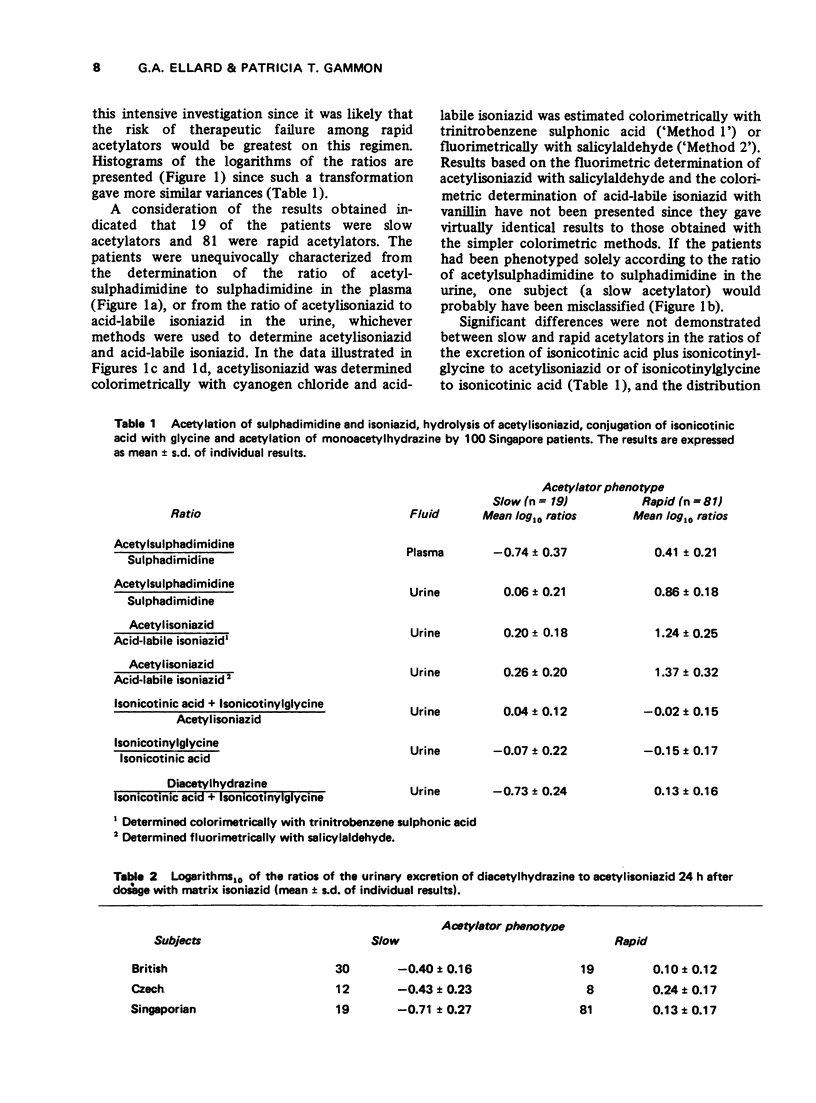
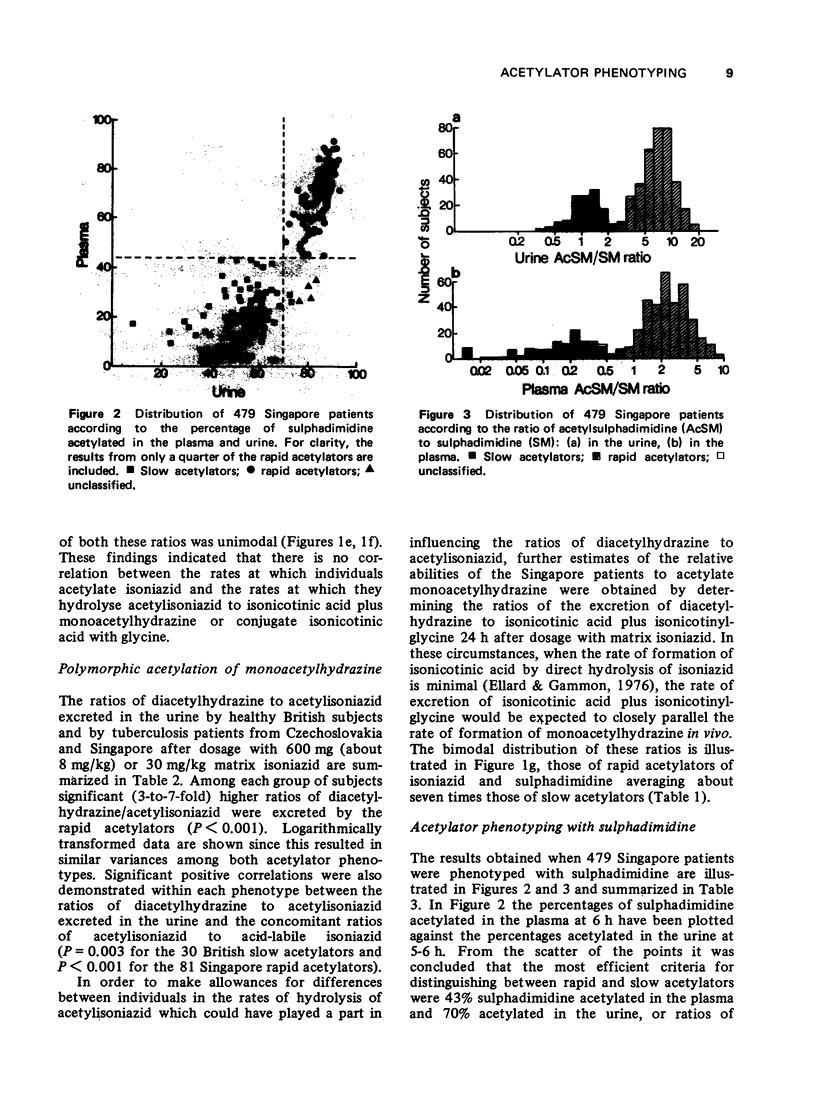
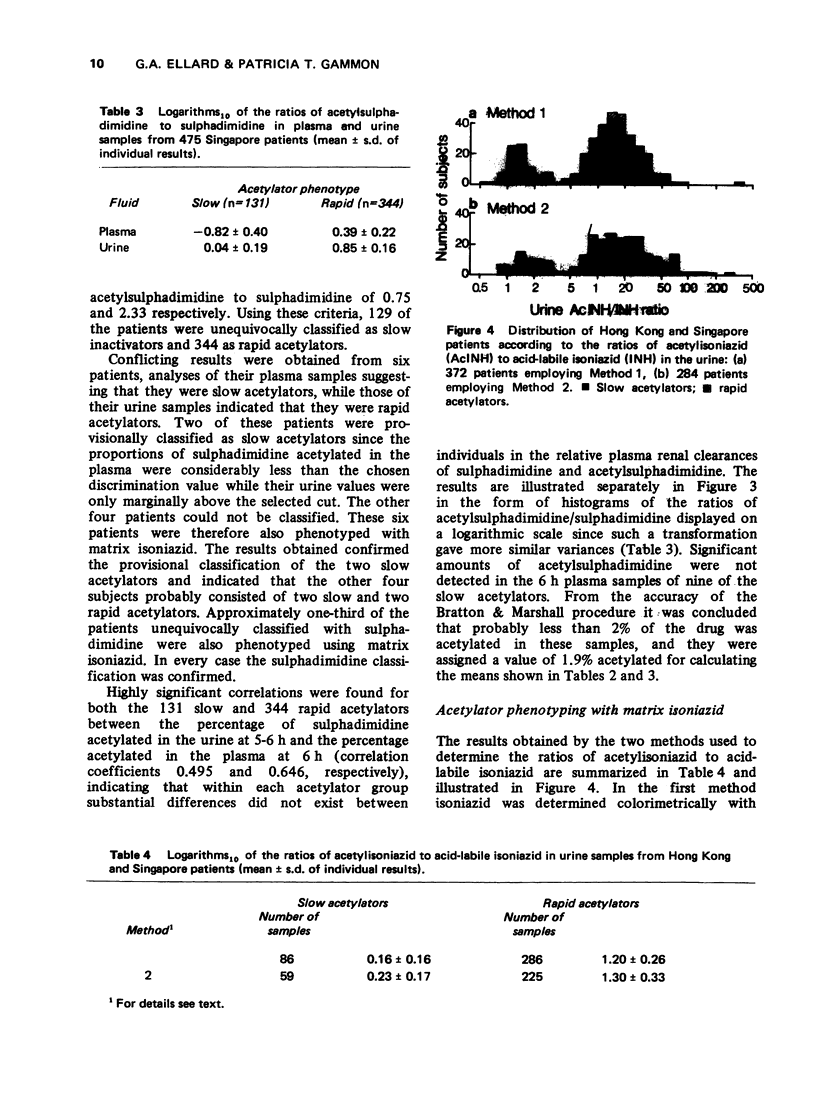
1. The acetylator phenotype of over 600 pulmonary tuberculosis patients treated with intermittent isoniazid-containing regimens in two controlled clinical trials was determined using either sulphadimidine or a slow-release isoniazid formulation. 2. Both methods unequivocally classified over 99% of the patients as being either slow or rapid acetylators. 3. Rapid and slow acetylation did not differ in their ability of hydrolyse acetylisoniazid to isonicotonic acid plus monoacetylhydrazine, or to conjugate isonicotinic acid with glycine. 4. Rapid acetylators acetylated the monoacetylhydrazine liberated in vivo more rapidly than slow acetylators, demonstrating that this compound is also polymorphologically acetylated in man. 5. The acetylator phenotype of the patients was without prognostic significance when they were treated on a twice-weekly basis with isoniazid plus streptomycin plus pyraziniamide, or with isoniazid plus rifampicin. However, when patients were treated once every week for 12 months with isoniazid plus rifampicin, 5% of the rapid acetylators had an unsatisfactory response as contrasted to the complete success of the treatment in the slow acetylators.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dymond L. C., Russell D. W. Rapid determination of isonicotinic acid hydrazide in whole blood with 2,4,6-trinitrobenzenesulphonic acid. Clin Chim Acta. 1970 Mar;27(3):513–520. doi: 10.1016/0009-8981(70)90306-2. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976 Apr;4(2):83–113. doi: 10.1007/BF01086149. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Polansky F., Víznerová A., Havlík I., Fox W. Further studies on the pharmacology of a slow-release matrix preparation of isoniazid (Smith and Nephew HS 82) of potential use in the intermittent treatment of tuberculosis. Tubercle. 1973 Mar;54(1):57–66. doi: 10.1016/0041-3879(73)90015-9. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Titinen H. Determination of the acetylator phenotype using matrix isoniazid. Tubercle. 1975 Sep;56(3):203–209. doi: 10.1016/0041-3879(75)90053-7. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Wallace S. M. The determination of isoniazid and its metabolites acetylisoniazid, monoacetylhydrazine, diacetylhydrazine, isonicotinic acid and isonicotinylglycine in serum and urine. Biochem J. 1972 Feb;126(3):449–458. doi: 10.1042/bj1260449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard G. A. Variations between individuals and populations in the acetylation of isoniazid and its significance for the treatment of pulmonary tuberculosis. Clin Pharmacol Ther. 1976 May;19(5 Pt 2):610–625. doi: 10.1002/cpt1976195part2610. [DOI] [PubMed] [Google Scholar]
- Evans D. A. An improved and simplified method of detecting the acetylator phenotype. J Med Genet. 1969 Dec;6(4):405–407. doi: 10.1136/jmg.6.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A. Genetic variations in the acetylation of isoniazid and other drugs. Ann N Y Acad Sci. 1968 Jul 31;151(2):723–733. doi: 10.1111/j.1749-6632.1968.tb48255.x. [DOI] [PubMed] [Google Scholar]
- Fox W. General considerations in the choice and management of regimens of chemotherapy for pulmonary tuberculosis. Bull Int Union Tuberc. 1972 Feb;47:49–67. [PubMed] [Google Scholar]
- Fox W., Mitchison D. A. Short-course chemotherapy for pulmonary tuberculosis. Am Rev Respir Dis. 1975 Mar;111(3):325–353. doi: 10.1164/arrd.1975.111.3.325. [DOI] [PubMed] [Google Scholar]
- Kailasam S., Immanuel C., Nair N. G., Radhakrishma S., Tripathy S. P. Classification of subjects as slow or rapid inactivators of isoniazid oral administration of a slow-release preparation of isoniazid and determination of the ratio of acetylisoniazid to isoniazid in urine. Indian J Med Res. 1975 Feb;63(2):323–328. [PubMed] [Google Scholar]
- La Du B. N. Isoniazid and psuedocholinesterase polymorphisms. Fed Proc. 1972 Jul-Aug;31(4):1276–1285. [PubMed] [Google Scholar]
- Mitchell J. R., Thorgeirsson U. P., Black M., Timbrell J. A., Snodgrass W. R., Potter W. Z., Jollow H. R., Keiser H. R. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975 Jul;18(1):70–79. doi: 10.1002/cpt197518170. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Zimmerman H. J., Ishak K. G., Thorgeirsson U. P., Timbrell J. A., Snodgrass W. R., Nelson S. D. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976 Feb;84(2):181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- Peters J. H. Genetic factors in relation to drugs. Annu Rev Pharmacol. 1968;8:427–452. doi: 10.1146/annurev.pa.08.040168.002235. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Miller K. S., Brown P. Studies on the metabolic basis for the genetically determined capacities for isoniazid inactivation in man. J Pharmacol Exp Ther. 1965 Nov;150(2):298–304. [PubMed] [Google Scholar]
- Rao K. V., Mitchison D. A., Nair N. G., Prema K., Tripathy S. P. Sulphadimidine acetylation test for classification of patients as slow or rapid inactivators of isoniazid. Br Med J. 1970 Aug 29;3(5721):495–497. doi: 10.1136/bmj.3.5721.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNAHARA S., URANO M., LIN H. T., CHEG T. J., JARUMILINDA A. FURTHER OBSERVATIONS ON TRIMODALITY OF FREQUENCY DISTRIBUTION CURVE OF BIOLOGICALLY ACTIVE ISONIAZID BLOOD LEVELS AND "CLINE" INFREQUENCIES OF ALLELES CONTROLLING ISONIAZID INACTIVATION. Acta Tuberc Pneumol Scand. 1963;43:181–195. [PubMed] [Google Scholar]
- Sarma G. R., Immanuel C., Kailasam S., Kannapiran M., Nair N. G., Radhakrishna S. A modified method for the estimation of acetylisoniazid in urine. Indian J Med Res. 1974 Jun;62(6):945–952. [PubMed] [Google Scholar]
- Scott E. M., Wright R. C., Weaver D. D. The discrimination of phenotypes for rate of disappearance of isonicotinoyl hydrazide from serum. J Clin Invest. 1969 Jul;48(7):1173–1176. doi: 10.1172/JCI106081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman P., Eidus L., Tripathy S. P. Method for the estimation of acetylisoniazid in urine. Tubercle. 1968 Jun;49(2):210–216. doi: 10.1016/0041-3879(68)90023-8. [DOI] [PubMed] [Google Scholar]
- Víznerová A., Slavíková Z., Ellard G. A. The determination of the acetylator phenotype of tuberculosis patients in Czechoslovakia using sulphadimidine. Tubercle. 1973 Mar;54(1):67–71. doi: 10.1016/0041-3879(73)90016-0. [DOI] [PubMed] [Google Scholar]


