Abstract
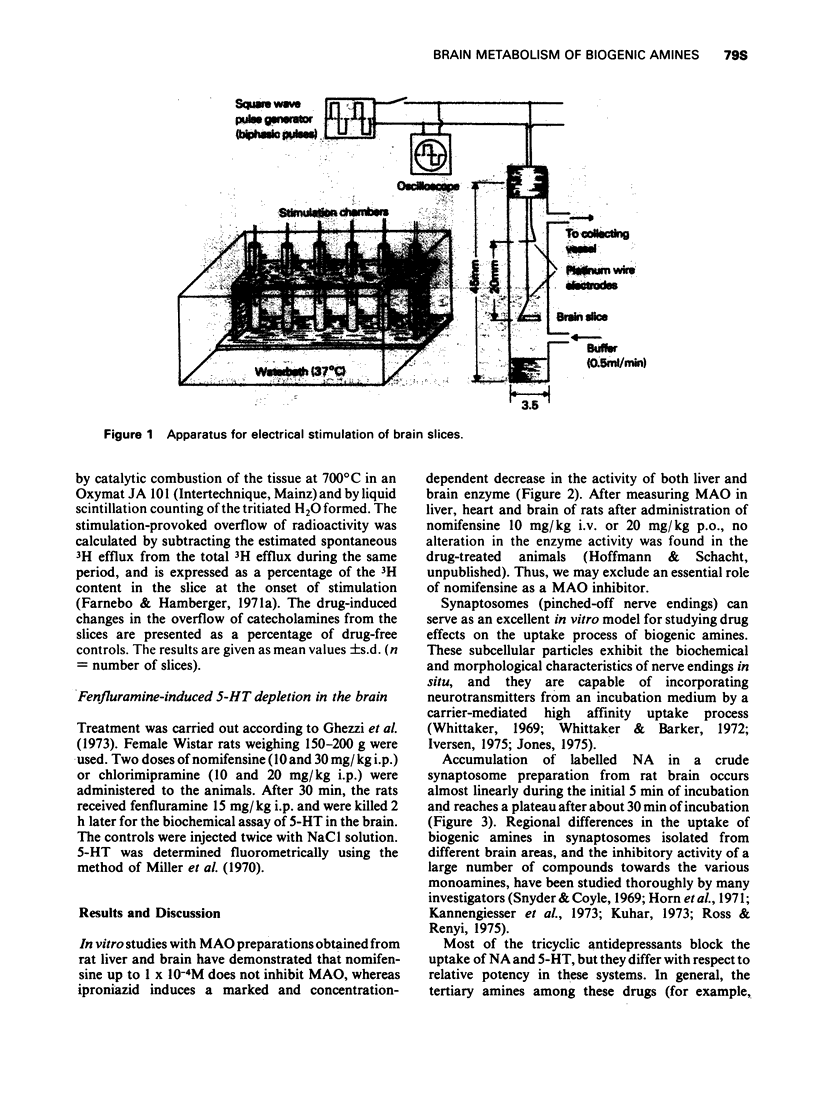
1 In vitro models (synaptosones, electrically stimulated brain slices and monoamine oxidase (MAO) preparations have been used to identify the sites of action of nomifensine on brain monoamine metabolism.
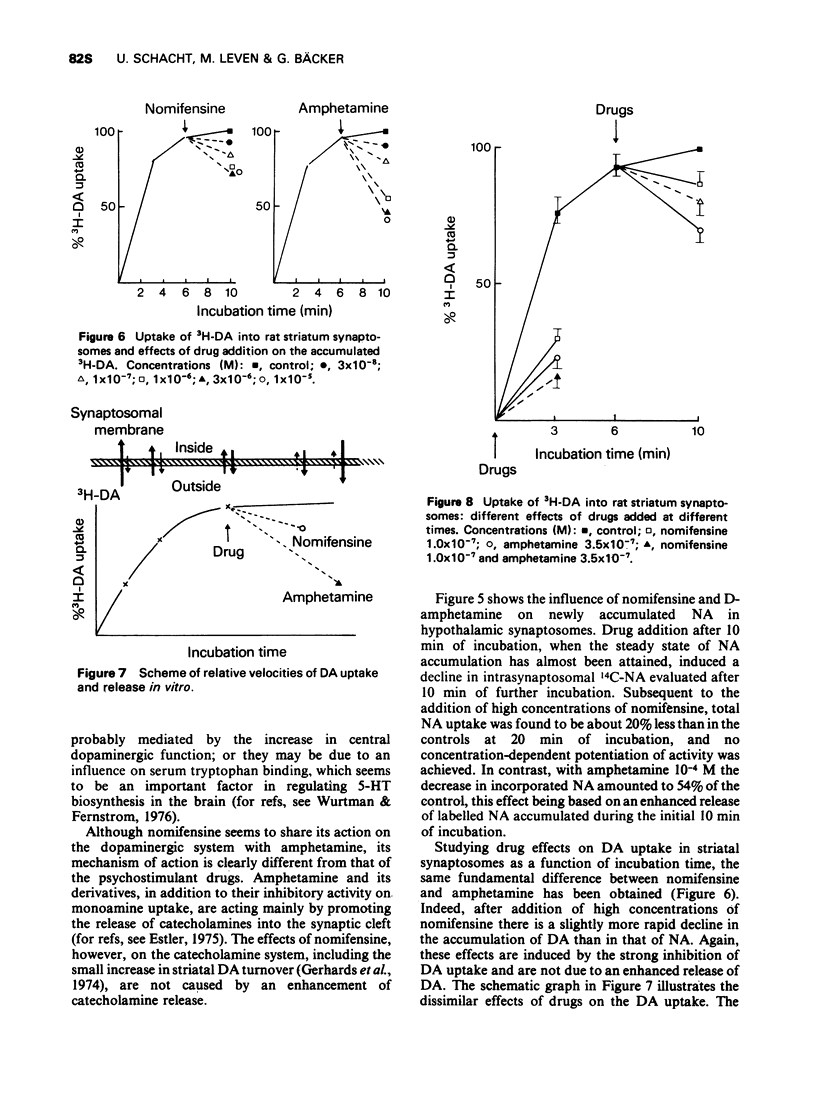
2 Nomifensine potentiates neurotransmission in noradrenergic and dopaminergic synapses by blocking catecholamine uptake.
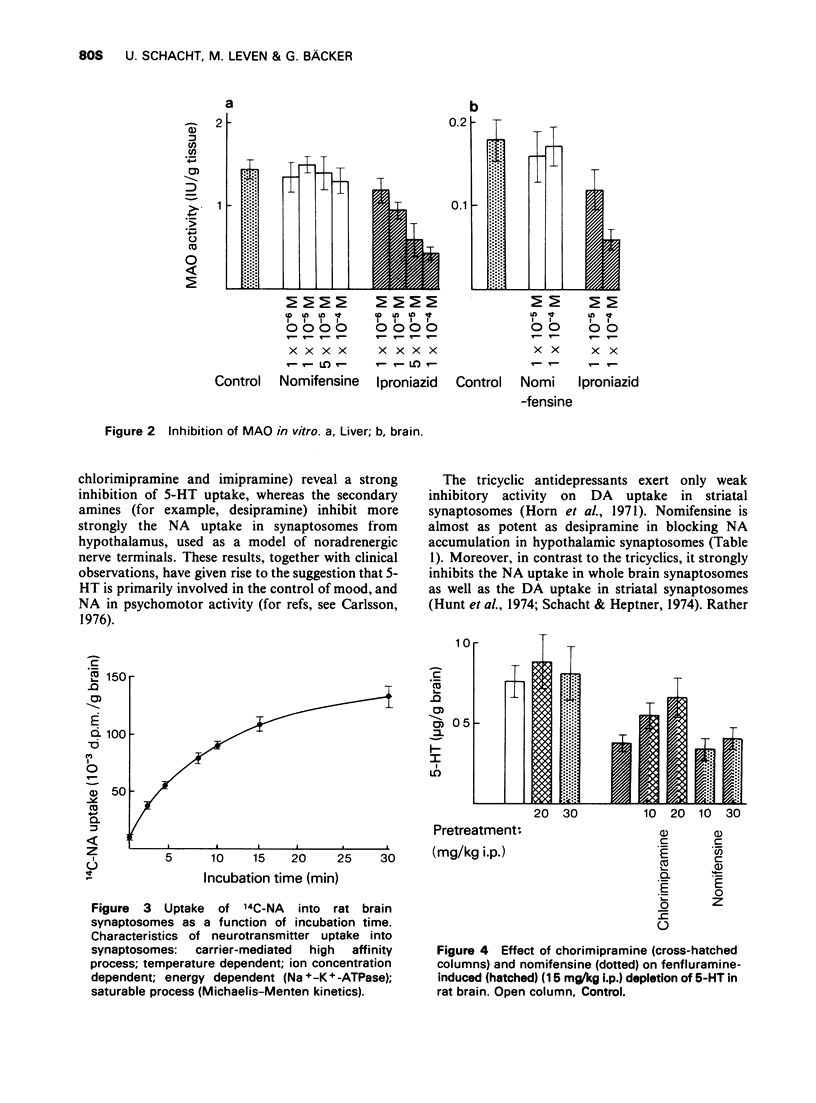
3 Nomifensine does not inhibit MAO and does not enhance the release of biogenic amines.
4 Studies on electrically stimulated brain slices suggest that nomifensine does not directly stimulate dopamine (DA) nor noradrenaline (NA) receptor sites.
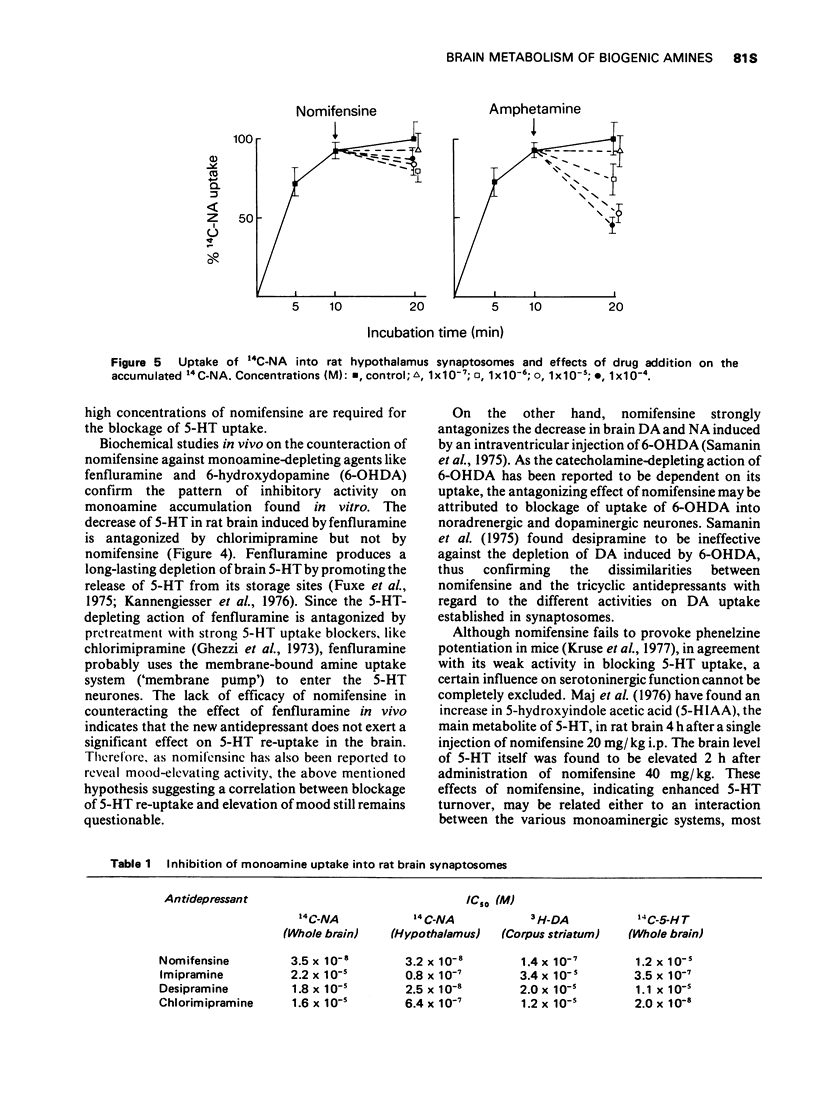
5 5-Hydroxytryptamine (5-HT) uptake is not inhibited at `therapeutic' concentrations of nomifensine.
6 Nomifensine differs from the tricyclic antidepressants by virtue of its effect on the dopaminergic system.
Full text
PDF

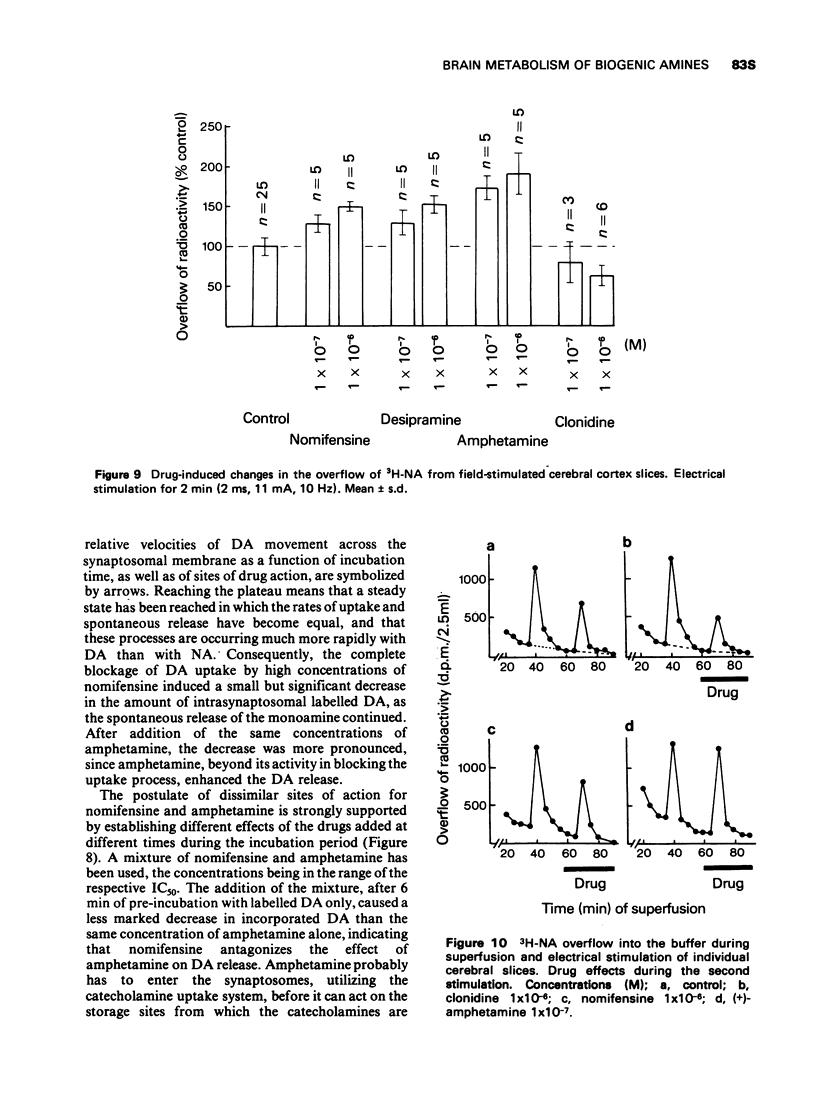
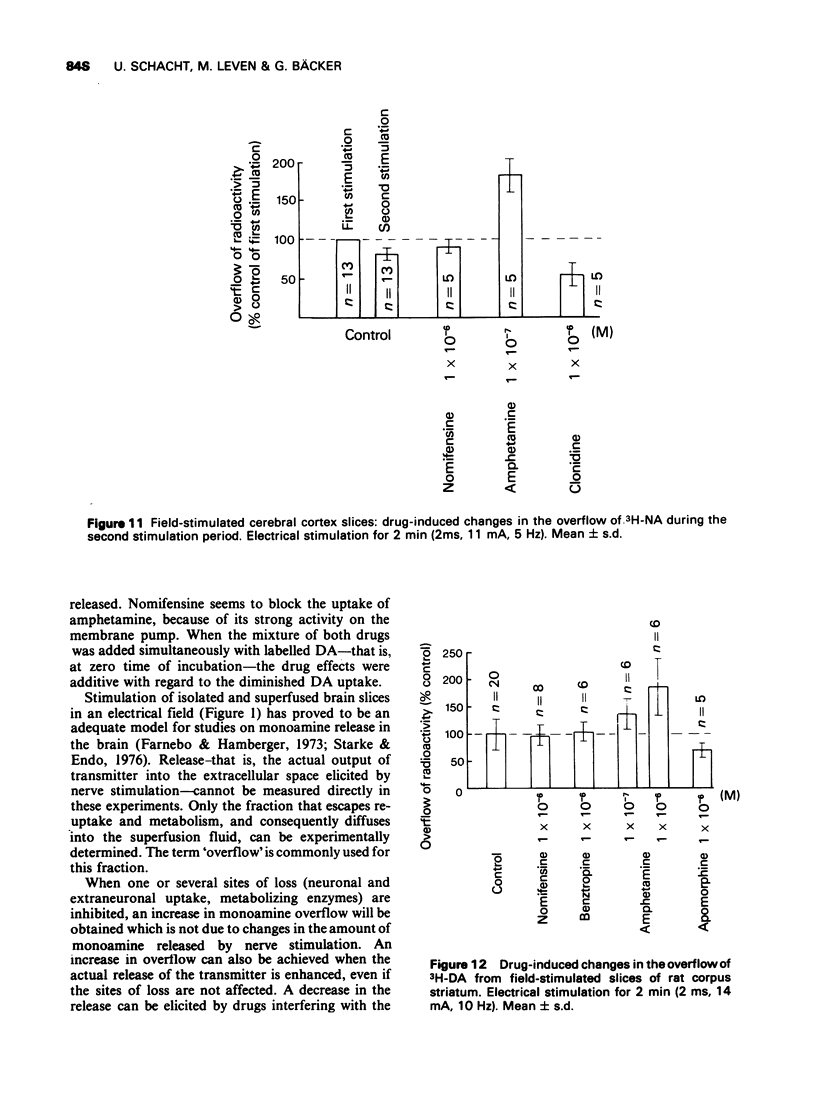



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkmayer W., Riederer P. Biochemical post-mortem findings in depressed patients. J Neural Transm. 1975;37(2):95–109. doi: 10.1007/BF01663627. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The contribution of drug research to investigating the nature of endogenous depression. Pharmakopsychiatr Neuropsychopharmakol. 1976 Jan;9(1):2–10. [PubMed] [Google Scholar]
- Costall B., Kelly D. M., Naylor R. J. Nomifensine: a potent dopaminergic agonist of antiparkinson potential. Psychopharmacologia. 1975;41(2):153–164. doi: 10.1007/BF00421073. [DOI] [PubMed] [Google Scholar]
- Estler C. J. Effect of amphetamine-type psychostimulants on brain metabolism. Adv Pharmacol Chemother. 1975;13:305–357. doi: 10.1016/s1054-3589(08)60234-3. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of 3 H-monoamines from field stimulated rat brain slices. Acta Physiol Scand Suppl. 1971;371:35–44. doi: 10.1111/j.1748-1716.1971.tb05213.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Farnebo L. O., Hamberger B., Ogren S. O. On the in vivo and in vitro actions of fenfluramine and its derivatives on central monoamine neurons, especially 5-hydroxytryptamine neurons, and their relation to the anorectic activity of fenfluramine. Postgrad Med J. 1975;51 (Suppl 1):35–45. [PubMed] [Google Scholar]
- Gerhards H. J., Carenzi A., Costa E. Effect of nomifensine on motor activity, dopamine turnover rate and cyclic 3',5;-adenosine monophosphate concentrations of rat striatum. Naunyn Schmiedebergs Arch Pharmacol. 1974;286(1):49–63. doi: 10.1007/BF00499104. [DOI] [PubMed] [Google Scholar]
- Ghezzi D., Samanin R., Bernasconi S., Tognoni G., Gerna M., Garattini S. Effect of thymoleptics on fenfluramine-induced depletion of brain serotonin in rats. Eur J Pharmacol. 1973 Nov;24(2):205–210. doi: 10.1016/0014-2999(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Hendley E. D., Snyder S. H., Fauley J. J., LaPidus J. B. Stereoselectivity of catecholamine uptake by brain synaptosomes: studies with ephedrine, methylphenidate and phenyl-2-piperidyl carbinol. J Pharmacol Exp Ther. 1972 Oct;183(1):103–116. [PubMed] [Google Scholar]
- Hoffmann I. 8-Amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline, a new antidepressant. Arzneimittelforschung. 1973 Jan;23(1):45–50. [PubMed] [Google Scholar]
- Horn A. S., Coyle J. T., Snyder S. H. Catecholamine uptake by synaptosomes from rat brain. Structure-activity relationships of drugs with differential effects on dopamine and norepinephrine neurons. Mol Pharmacol. 1971 Jan;7(1):66–80. [PubMed] [Google Scholar]
- Hunt P., Kannengiesser M., Raynaud J. Nomifensine: a new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J Pharm Pharmacol. 1974 May;26(5):370–371. doi: 10.1111/j.2042-7158.1974.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Kannengiesser M. H., Hunt P., Raynaud J. P. An in vitro model for the study of psychotropic drugs and as a criterion of antidepressant activity. Biochem Pharmacol. 1973 Jan 1;22(1):73–84. doi: 10.1016/0006-2952(73)90256-6. [DOI] [PubMed] [Google Scholar]
- Kruse H., Hoffmann I., Gerhards H. J., Leven M., Schacht U. Pharmacological and biochemical studies with three metabolites of nomifensine. Psychopharmacology (Berl) 1977 Jan 31;51(2):117–123. doi: 10.1007/BF00431726. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. Effects of catecholamine releasing agents on synaptosomal dopamine biosynthesis: multiple pools of dopamine or multiple forms of tyrosine hydroxylase. Neuropharmacology. 1975 Jan;14(1):1–10. doi: 10.1016/0028-3908(75)90060-x. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J. Neurotransmitter uptake: a tool in identifying neurotransmitter-specific pathways. Life Sci. 1973 Dec 16;13(12):1623–1634. doi: 10.1016/0024-3205(73)90110-0. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Maj J., Kapturkiewicz Z., Michaluk J. Uber die zentrale Wirkung von Nomifensin. Arzneimittelforschung. 1976;26(6):1109–1111. [PubMed] [Google Scholar]
- Miller F. P., Cox R. H., Jr, Snodgrass W. R., Maickel R. P. Comparative effects of p-chloroamphetamine and p-chloro-N-methylamphetamine on rat brain norepinephrine, serotonin and 5-hydroxyindole-3-acetic acid. Biochem Pharmacol. 1970 Feb;19(2):435–442. doi: 10.1016/0006-2952(70)90199-1. [DOI] [PubMed] [Google Scholar]
- Samanin R., Bernasconi S., Garattini S. The effect of nomifensine on the depletion of brain serotonin and catecholamines induced respectively by fenfluramine and 6-hydroxydopamine in rats. Eur J Pharmacol. 1975 Dec;34(2):377–380. doi: 10.1016/0014-2999(75)90266-6. [DOI] [PubMed] [Google Scholar]
- Schacht U., Heptner W. Effect of nomifensine (HOE 984), a new antidepressant, on uptake of noradrenaline and serotonin and on release of noradrenaline in rat brain synaptosomes. Biochem Pharmacol. 1974 Dec 15;23(24):3413–3422. doi: 10.1016/0006-2952(74)90344-x. [DOI] [PubMed] [Google Scholar]
- Schildkraut J. J. Biogenic amines and affective disorders. Annu Rev Med. 1974;25(0):333–348. doi: 10.1146/annurev.me.25.020174.002001. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Coyle J. T. Regional differences in H3-norepinephrine and H3-dopamine uptake into rat brain homogenates. J Pharmacol Exp Ther. 1969 Jan;165(1):78–86. [PubMed] [Google Scholar]
- Starke K., Endo T. Presynaptic alpha-adrenoceptors. Gen Pharmacol. 1976 Oct;7(5):307–312. doi: 10.1016/0306-3623(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Fernstrom J. D. Control of brain neurotransmitter synthesis by precursor availability and nutritional state. Biochem Pharmacol. 1976 Aug 1;25(15):1691–1696. doi: 10.1016/0006-2952(76)90400-7. [DOI] [PubMed] [Google Scholar]
- van Praag H. M., Korf J. Central monoamine deficiency in depressions: causative of secondary phenomenon? Pharmakopsychiatr Neuropsychopharmakol. 1975 Sep;8(5):322–326. doi: 10.1055/s-0028-1094463. [DOI] [PubMed] [Google Scholar]


