Abstract
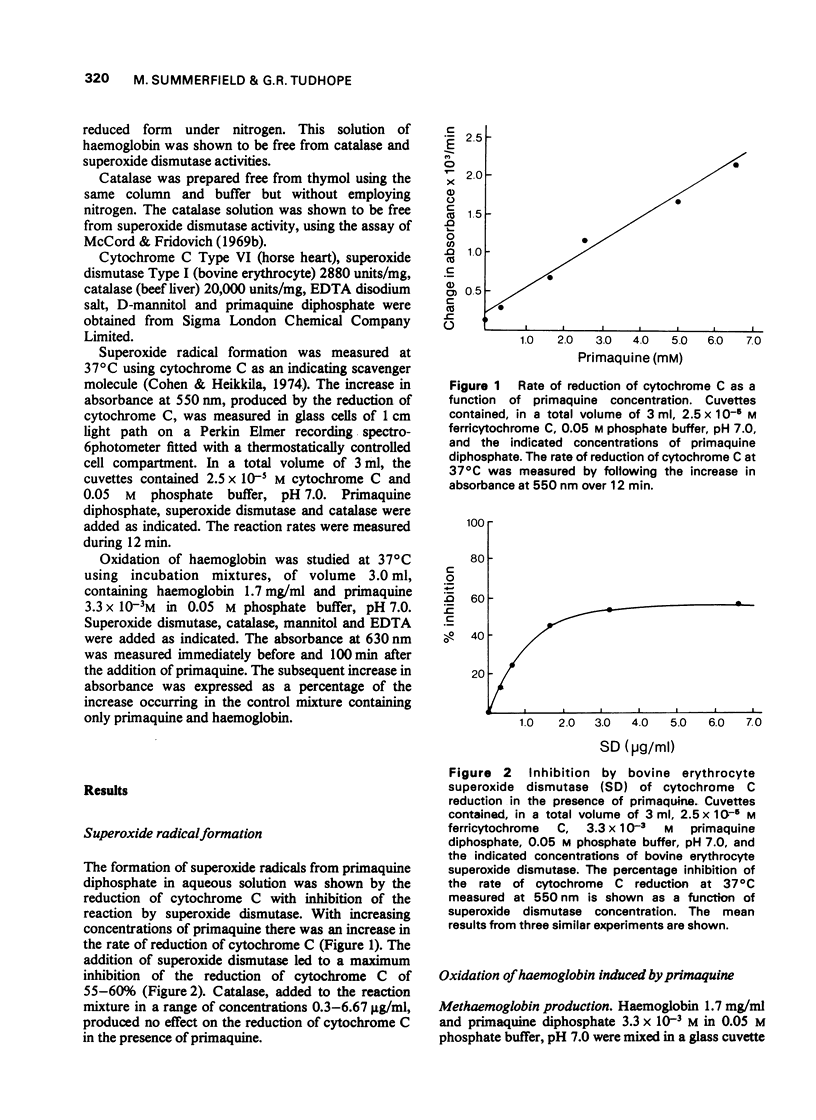
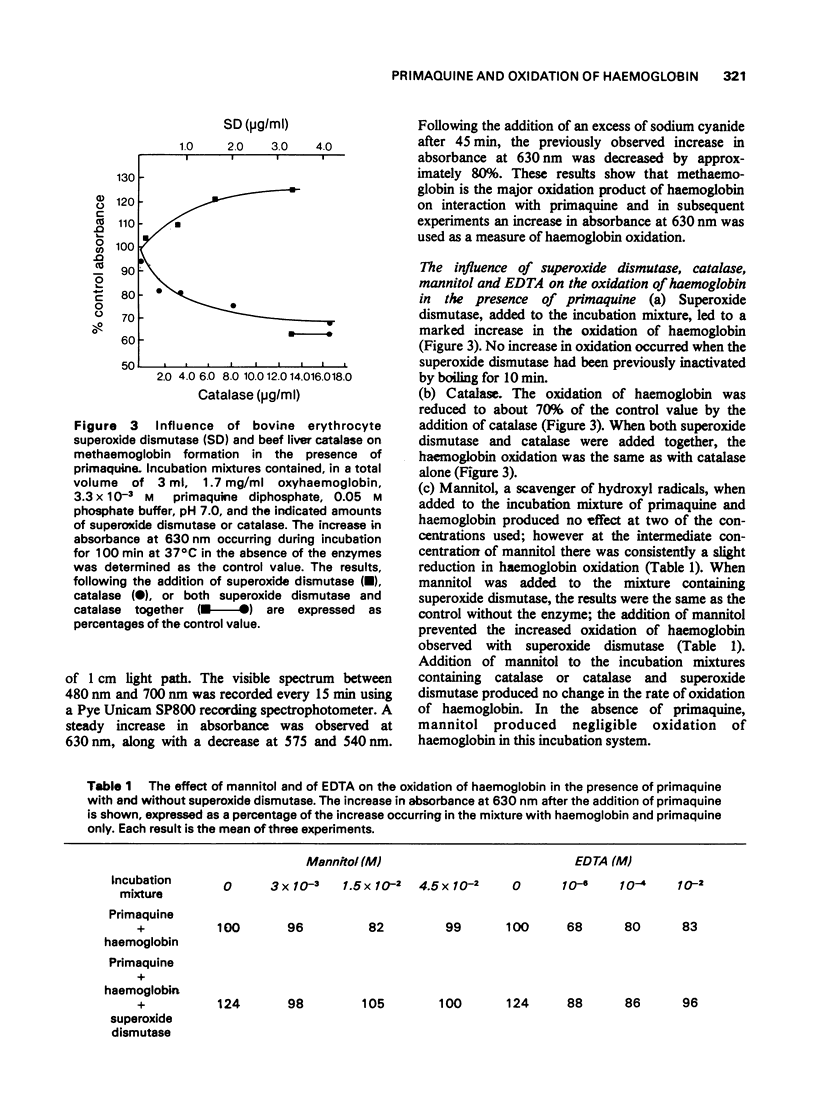
1. The production of superoxide radicals from primaquine diphosphate in aqueous solution has been demonstrated, using as indicator the reduction of cytochrome C with inhibition of the reaction by superoxide dismutase. 2. Primaquine-mediated oxidation of haemoglobin to methaemoglobin was reduced by the addition of catalase and increased by superoxide dismutase. Mannitol, a hydroxyl radical scavenger, abolished the increase in methaemoglobin observed in the presence of superoxide dismutase. EDTA reduced the oxidation of haemoglobin with and without superoxide dismutase. 3. Although the oxidation of haemoglobin in the presence of primaquine includes the effects of hydrogen peroxide, superoxide and hydroxyl radicals and metal ions, the results indicate that hydrogen peroxide, rather than the superoxide radical, is the main oxidizing species. The increase in haemoglobin oxidation occurring with superoxide dismutase may result from the augmented rate of hydrogen peroxide formation from superoxide radicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E. The hemolytic effect of primaquine and related compounds: a review. Blood. 1959 Feb;14(2):103–139. [PubMed] [Google Scholar]
- Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969 Mar;21(1):73–103. [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. IN VIVO GENERATION OF H2O2 IN MOUSE ERYTHROCYTES BY HEMOLYTIC AGENTS. J Pharmacol Exp Ther. 1965 Jan;147:139–143. [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974 Apr 25;249(8):2447–2452. [PubMed] [Google Scholar]
- Fraser I. M., Vesell E. S. Effects of drugs and drug metabolites on erythrocytes from normal and glucose-6-phosphate dehydrogenase-deficient individuals. Ann N Y Acad Sci. 1968 Jul 31;151(2):777–794. doi: 10.1111/j.1749-6632.1968.tb48261.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Kiese M. The biochemical production of ferrihemoglobin-forming derivatives from aromatic amines, and mechanisms of ferrihemoglobin formation. Pharmacol Rev. 1966 Sep;18(3):1091–1161. [PubMed] [Google Scholar]
- Lynch R. E., Lee R., Cartwright G. E. Inhibition by superoxide dismutase of methemoglobin formation from oxyhemoglobin. J Biol Chem. 1976 Feb 25;251(4):1015–1019. [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969 Nov 25;244(22):6056–6063. [PubMed] [Google Scholar]
- Miller A., Smith H. C. The intracellular and membrane effects of oxidant agents on normal red cells. Br J Haematol. 1970 Sep;19(3):417–428. doi: 10.1111/j.1365-2141.1970.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry. 1976 Feb 10;15(3):681–687. doi: 10.1021/bi00648a036. [DOI] [PubMed] [Google Scholar]
- Tudhope G. R., Leece S. P. Red-cell catalase and the production of methaemoglobin, Heinz bodies and changes in osmotic fragility due to drugs. Acta Haematol. 1971;45(5):290–302. doi: 10.1159/000208639. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]


