Abstract
1 A single oral dose of 500 mg diflunisal was administered to control subjects and patients with varying degrees of renal insufficiency to estimate the disposition kinetics of this drug.
2 Diflunisal and the sum of its ester and ether glucuronides conjugates were measured fluorimetrically.
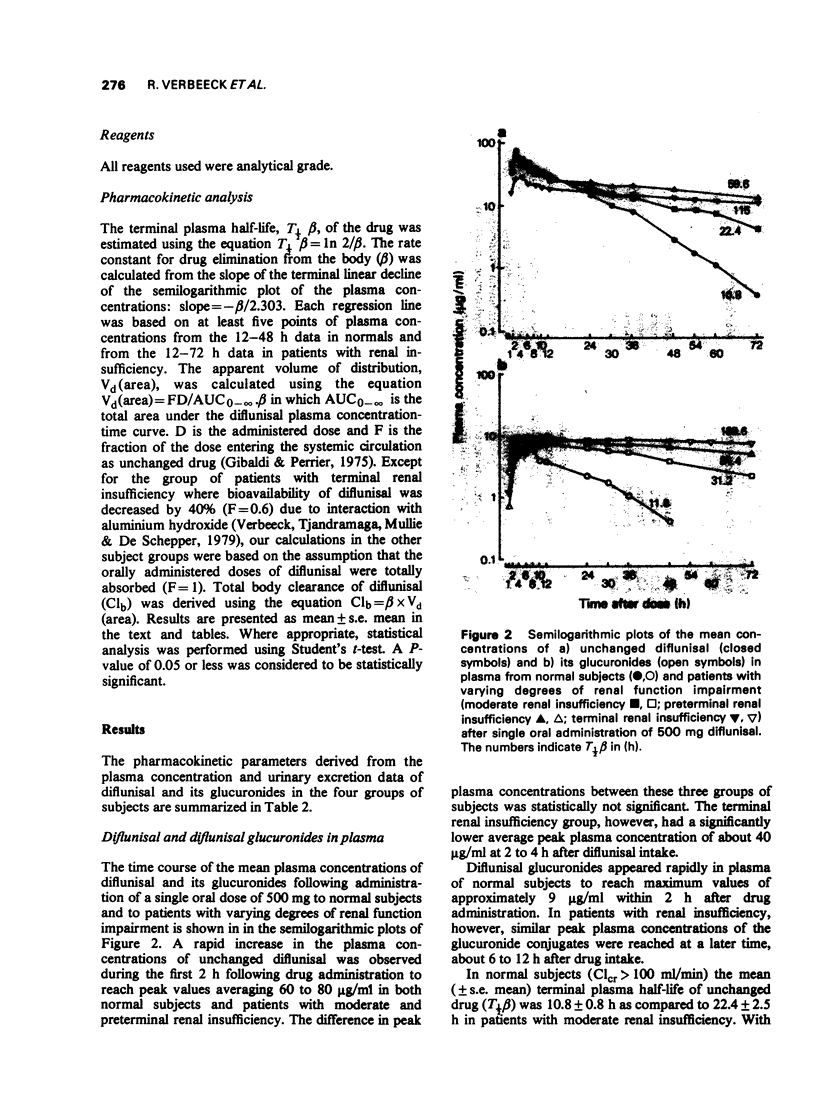
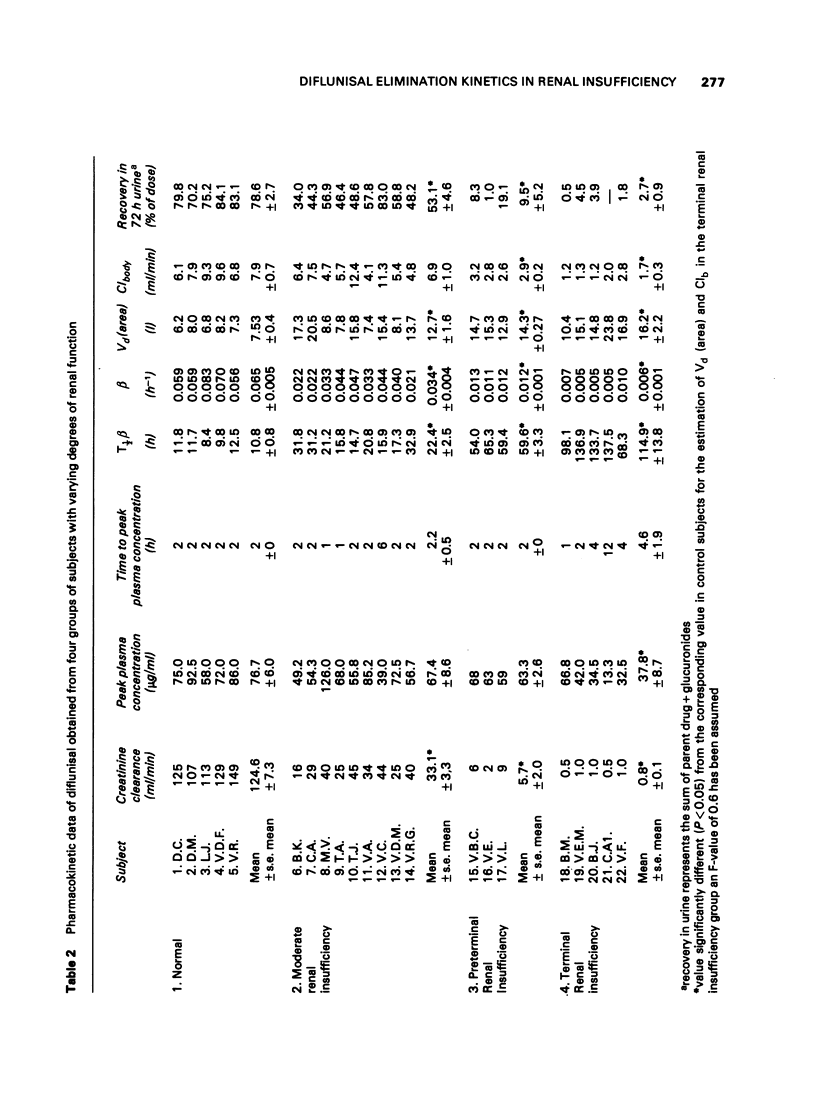
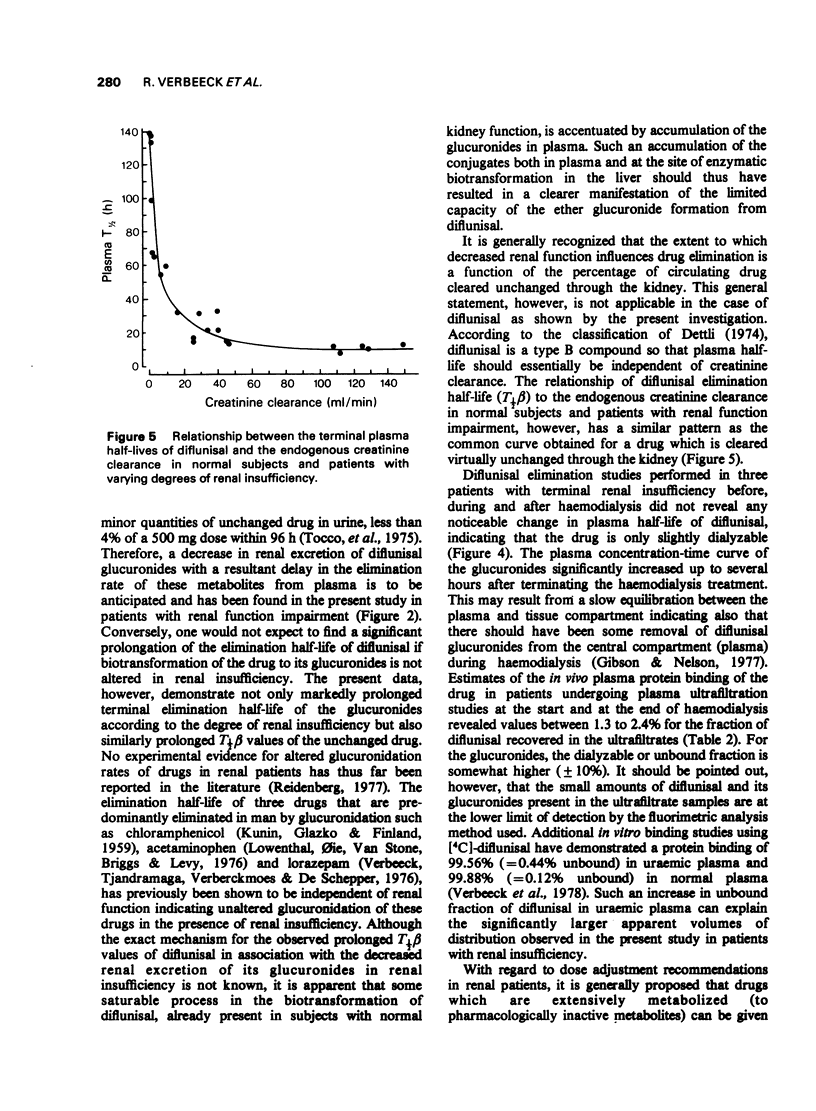
3 In normals terminal plasma half-lives (T½β) of diflunisal and its glucuronides were very similar: 10.8 h and 11.8 h respectively. The finding that plasma half-life was shortened with declining diflunisal plasma levels suggests capacity-limited elimination.
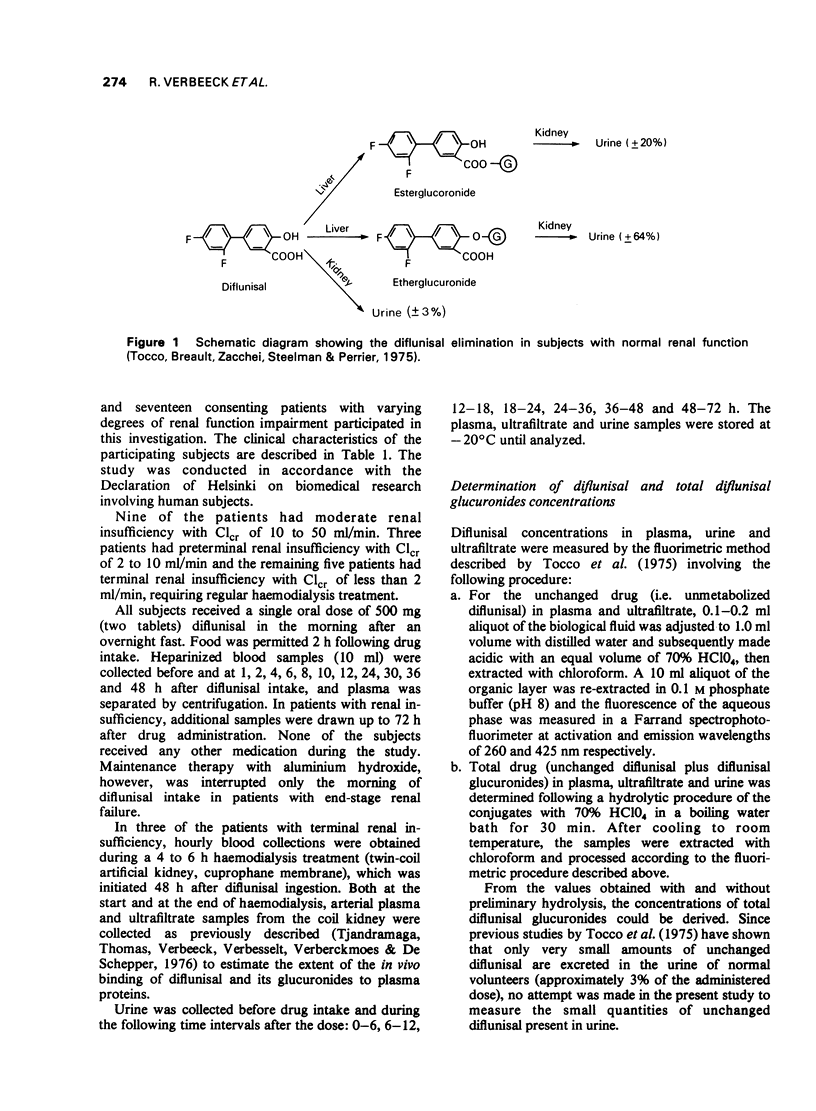
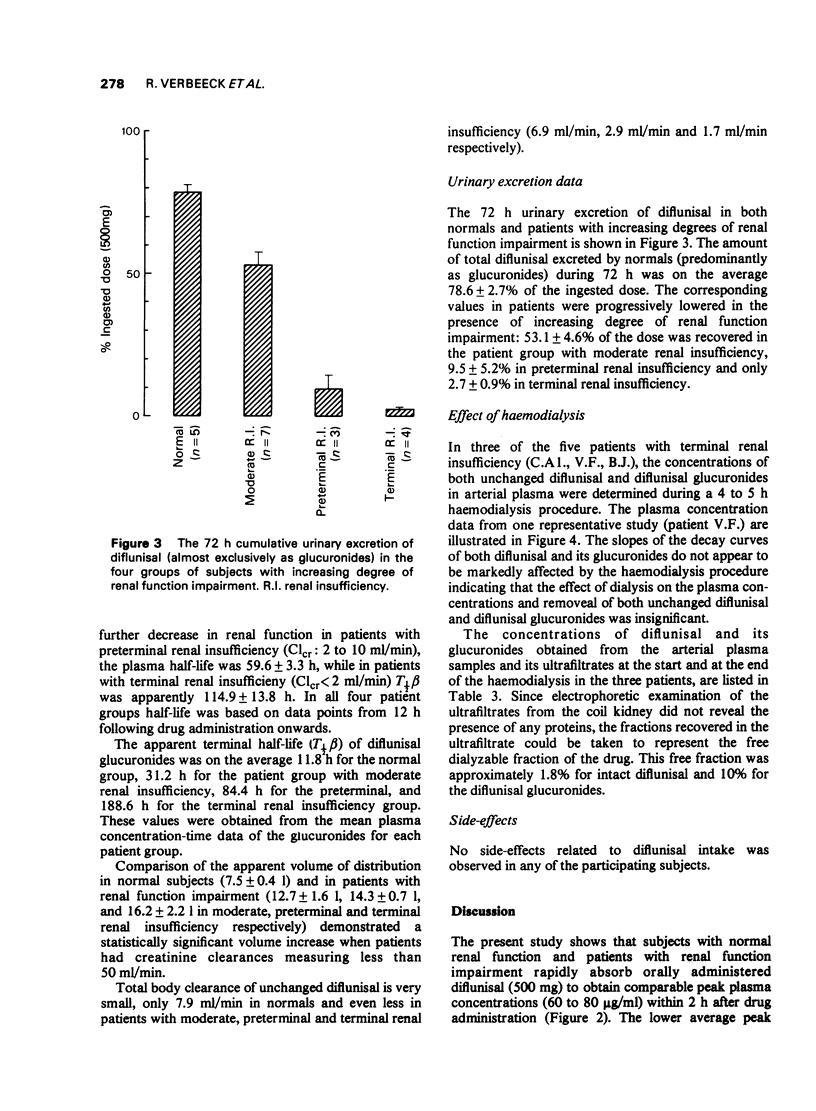
4 In subjects with normal renal function 78.6 ± 2.7% of the administered dose was recovered in 72 h urine, mainly as the glucuronide conjugates.
5 With increasing degree of renal function impairment T½β of diflunisal was progressively prolonged up to ten times normal probably due to slowed biotransformation. This was associated with increasing retention of the conjugated metabolites in plasma due to marked reduction of the urinary excretion of the glucuronide conjugates.
6 The apparent volume of distribution of diflunisal was very small in normals (7.3 ± 0.4 l) and was significantly increased in patients with renal insufficiency (up to 16.2 ± 2.2 l).
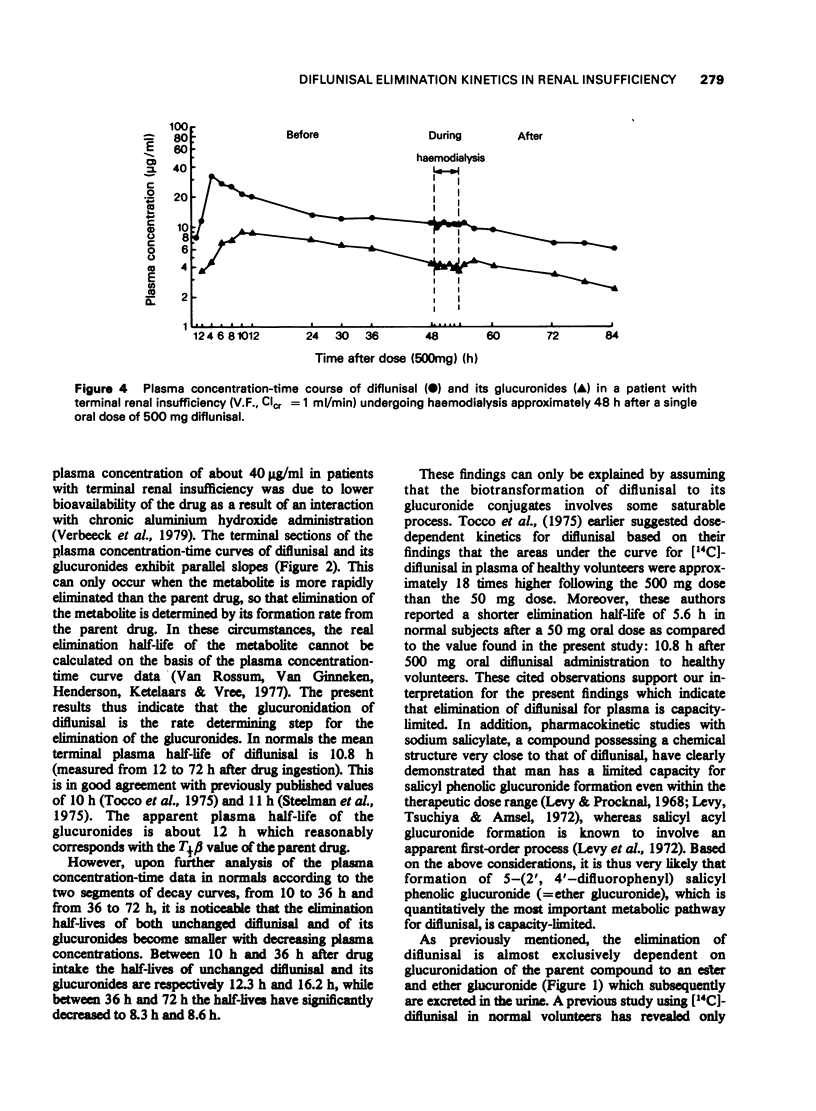
7 Diflunisal elimination studies performed during haemodialysis did not reveal any significant change in diflunisal plasma half-time. In vivo ultrafiltration studies during haemodialysis have shown that diflunisal is 98-99% plasma protein bound in uraemic patients.
8 The present study indicates that although diflunisal is primarily eliminated by biotransformation, T½β is prolonged in renal insufficiency and dose adjustment will accordingly be required in patients with renal function impairment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. M., Singer I., Coggins C. J. A guide to drug therapy in renal failure. JAMA. 1974 Dec 16;230(11):1544–1553. [PubMed] [Google Scholar]
- Cheigh J. S. Drug administration in renal failure. Am J Med. 1977 Apr;62(4):555–563. doi: 10.1016/0002-9343(77)90418-1. [DOI] [PubMed] [Google Scholar]
- Dettli L. Individualization of drug dosage in patients with renal disease. Med Clin North Am. 1974 Sep;58(5):977–985. doi: 10.1016/s0025-7125(16)32094-6. [DOI] [PubMed] [Google Scholar]
- Gibson T. B., Nelson H. A. Drug kinetics and artificial kidneys. Clin Pharmacokinet. 1977 Nov-Dec;2(6):403–426. doi: 10.2165/00003088-197702060-00002. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M., GLAZKO A. J., FINLAND M. Persistence of antibiotics in blood of patients with acute renal failure. II. Chloramphenicol and its metabolic products in the blood of patients with severe renal disease or hepatic cirrhosis. J Clin Invest. 1959 Sep;38:1498–1508. doi: 10.1172/JCI103928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G., Procknal J. A. Drug biotransformation interactions in man. I. Mutual inhibition in glucuronide formation of salicylic acid and salicylamide in man. J Pharm Sci. 1968 Aug;57(8):1330–1335. doi: 10.1002/jps.2600570811. [DOI] [PubMed] [Google Scholar]
- Levy G., Tsuchiya T., Amsel L. P. Limited capacity for salicyl phenolic glucuronide formation and its effect on the kinetics of salicylate elimination in man. Clin Pharmacol Ther. 1972 Mar-Apr;13(2):258–268. doi: 10.1002/cpt1972132258. [DOI] [PubMed] [Google Scholar]
- Lowenthal D. T., Oie S., Van Stone J. C., Briggs W. A., Levy G. Pharmacokinetics of acetaminophen elimination by anephric patients. J Pharmacol Exp Ther. 1976 Mar;196(3):570–578. [PubMed] [Google Scholar]
- Reidenberg M. M. The biotransformation of drugs in renal failure. Am J Med. 1977 Apr;62(4):482–485. doi: 10.1016/0002-9343(77)90401-6. [DOI] [PubMed] [Google Scholar]
- Tjandramaga T. B., Verbeeck R., Thomas J., Verbesselt R., Verberckmoes R., Schepper P. J. The effect of end-stage renal failure and haemodialysis on the elimination kinetics of sotalol. Br J Clin Pharmacol. 1976 Apr;3(2):259–265. doi: 10.1111/j.1365-2125.1976.tb00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocco D. J., Breault G. O., Zacchei A. G., Steelman S. L., Perrier C. V. Physiological disposition and metabolism of 5-(2',4'-difluorophenyl)salicyclic acid, a new salicylate. Drug Metab Dispos. 1975 Nov-Dec;3(6):453–466. [PubMed] [Google Scholar]
- Van Winzum C., Rodda B. Diflunisal: efficacy in postoperative pain. Br J Clin Pharmacol. 1977 Feb;4 (Suppl 1):39S–43S. doi: 10.1111/j.1365-2125.1977.tb04513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


