Abstract
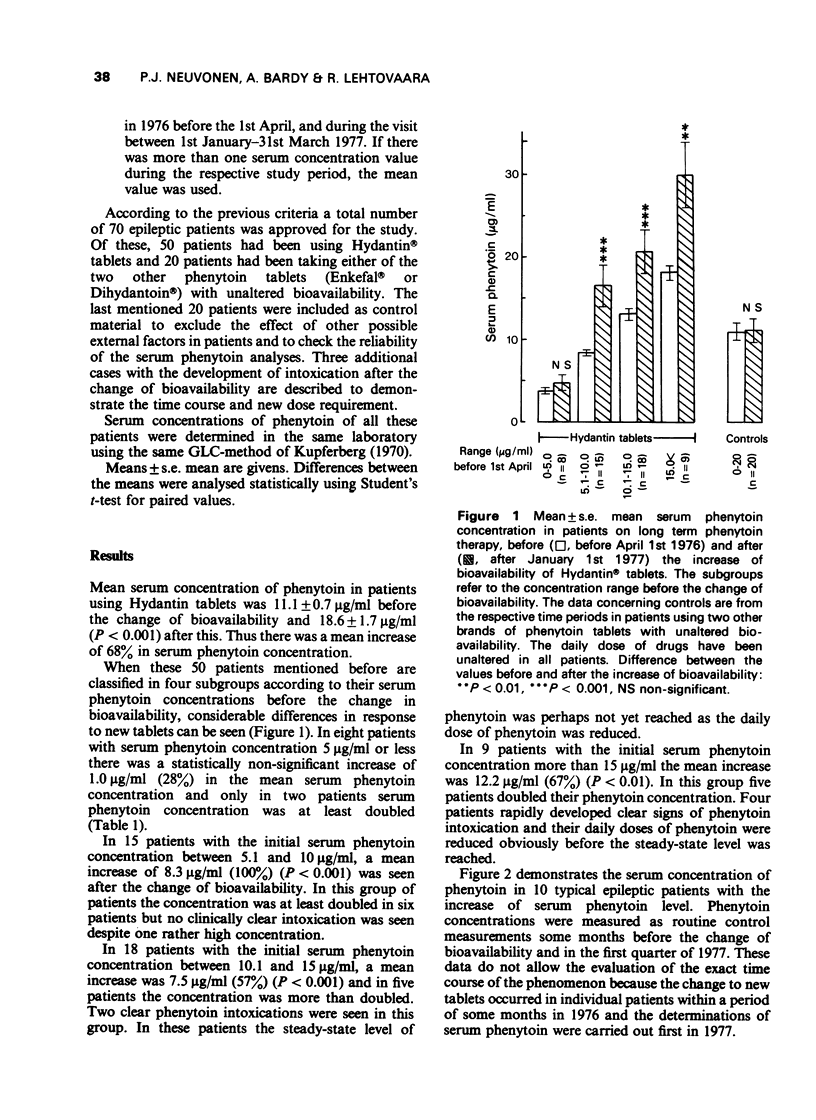
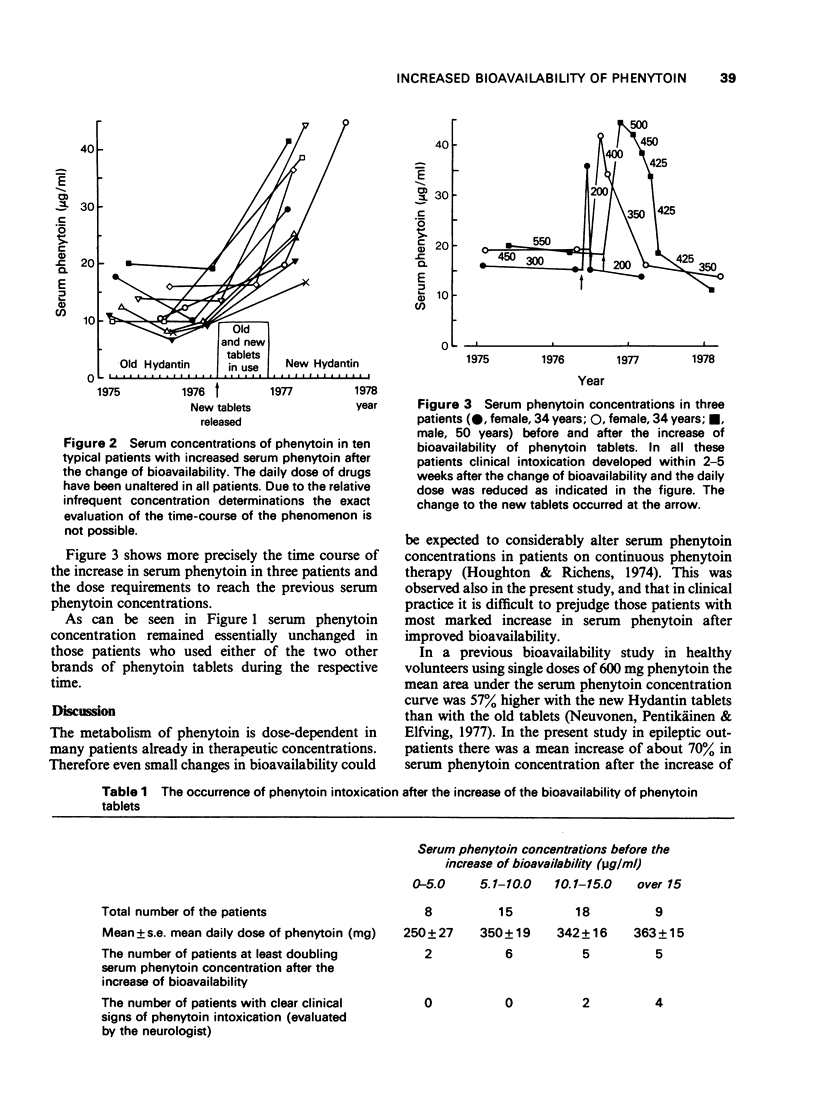
1. The bioavailability of a brand of phenytoin tablets used in Finland was improved in 1976. In the present retrospective study serum concentrations of phenytoin, measured before and after the change of bioavailability, are compared in 50 epileptic out-patients, who for various reasons used exactly the same dose of phenytoin tablets and of other drugs despite the increased bioavailability of phenytoin. 2. The mean increase of serum phenytoin steady-state concentration was about 70% after the change of bioavailability but there were considerable interindividual differences in the response. The mean increase in serum phenytoin was only 28% in patients with serum phenytoin concentrations 5 microgram/ml or less but the mean increase was 100% in patients with serum phenytoin between 5 and 10 microgram/ml. In patients with serum phenytoin concentrations more than 10 microgram/ml the mean increase in concentration was 60-80% after the improvement of bioavailability. However, in these groups of patients some clinically manifested phenytoin intoxications enforced the patients to the control and to dose reduction obviously before the steady-state concentration of phenytoin was reached. 3. On the basis of our experiences and those reported in the literature some proposals are presented to be considered when the bioavailability of phenytoin or of another drug with a narrow therapeutic range and a dose-dependent kinetics has to be changed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kupferberg H. J. Quantitative estimation of diphenylhydantoin, primidone and phenobarbital in plasma by gas-liquid chromatography. Clin Chim Acta. 1970 Aug;29(2):282–288. doi: 10.1016/0009-8981(70)90048-3. [DOI] [PubMed] [Google Scholar]
- Neuvonen P. J., Pentikäinen P. J., Elfving S. M. Factors affecting the bioavailability of phenytoin. Int J Clin Pharmacol Biopharm. 1977 Feb;15(2):84–89. doi: 10.103/00006450-11000-00005. [DOI] [PubMed] [Google Scholar]
- Pentikäinen P. J., Neuvonen P. J., Elfving S. M. Bioavailability of four brands of phenytoin tablets. Eur J Clin Pharmacol. 1975 Dec 19;9(2-3):213–218. doi: 10.1007/BF00614020. [DOI] [PubMed] [Google Scholar]
- Rambeck B., Boenigk H. E., Stenzel E. Bioavailability of three phenytoin preparations in healthy subjects and in epileptics. Eur J Clin Pharmacol. 1977 Dec 2;12(4):285–290. doi: 10.1007/BF00607428. [DOI] [PubMed] [Google Scholar]
- Tammisto P., Kauko K., Viukari M. Letter: Bioavailability of phenytoin. Lancet. 1976 Jan 31;1(7953):254–255. doi: 10.1016/s0140-6736(76)91382-9. [DOI] [PubMed] [Google Scholar]
- Tyrer J. H., Eadie M. J., Sutherland J. M., Hooper W. D. Outbreak of anticonvulsant intoxication in an Australian city. Br Med J. 1970 Oct 31;4(5730):271–273. doi: 10.1136/bmj.4.5730.271. [DOI] [PMC free article] [PubMed] [Google Scholar]


