Abstract
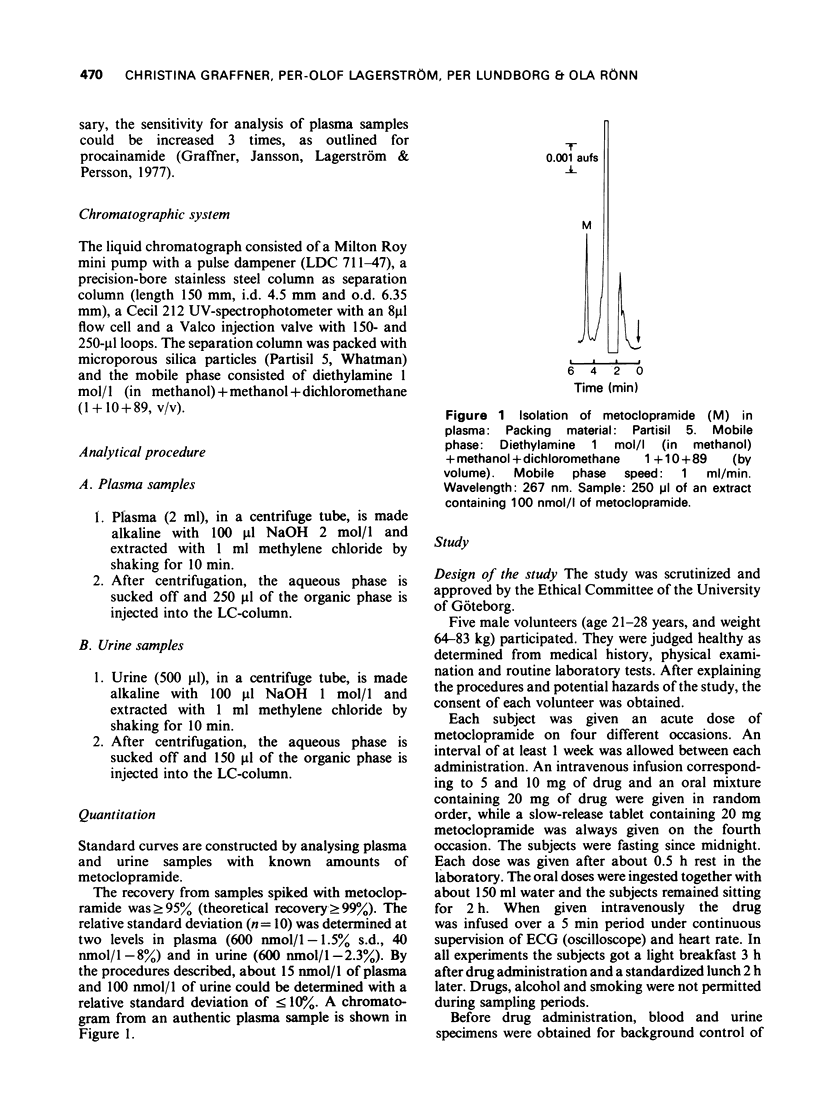
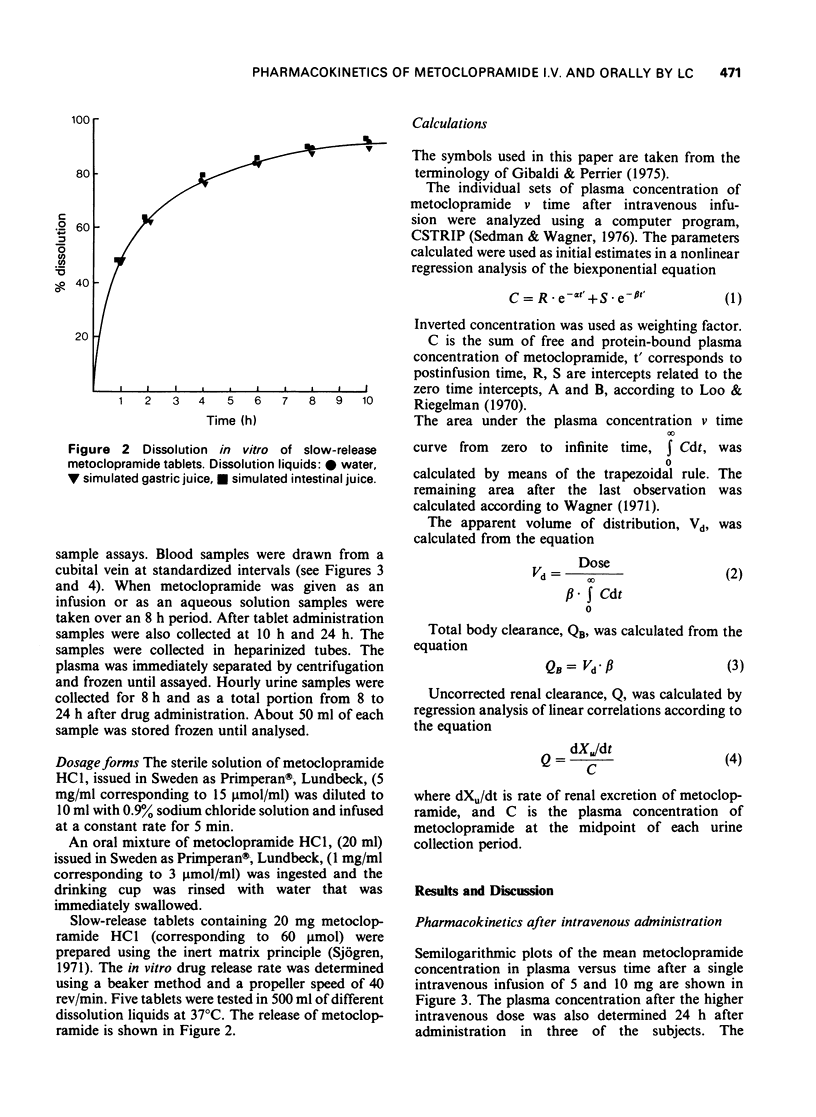
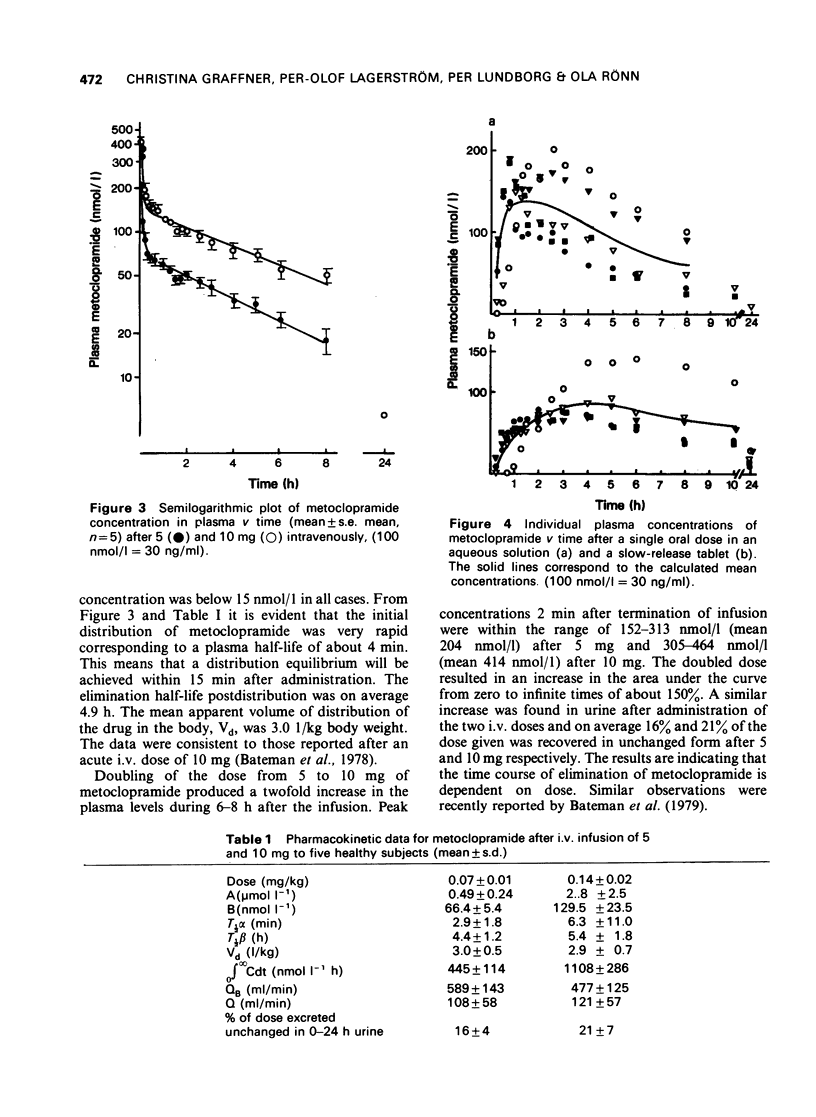
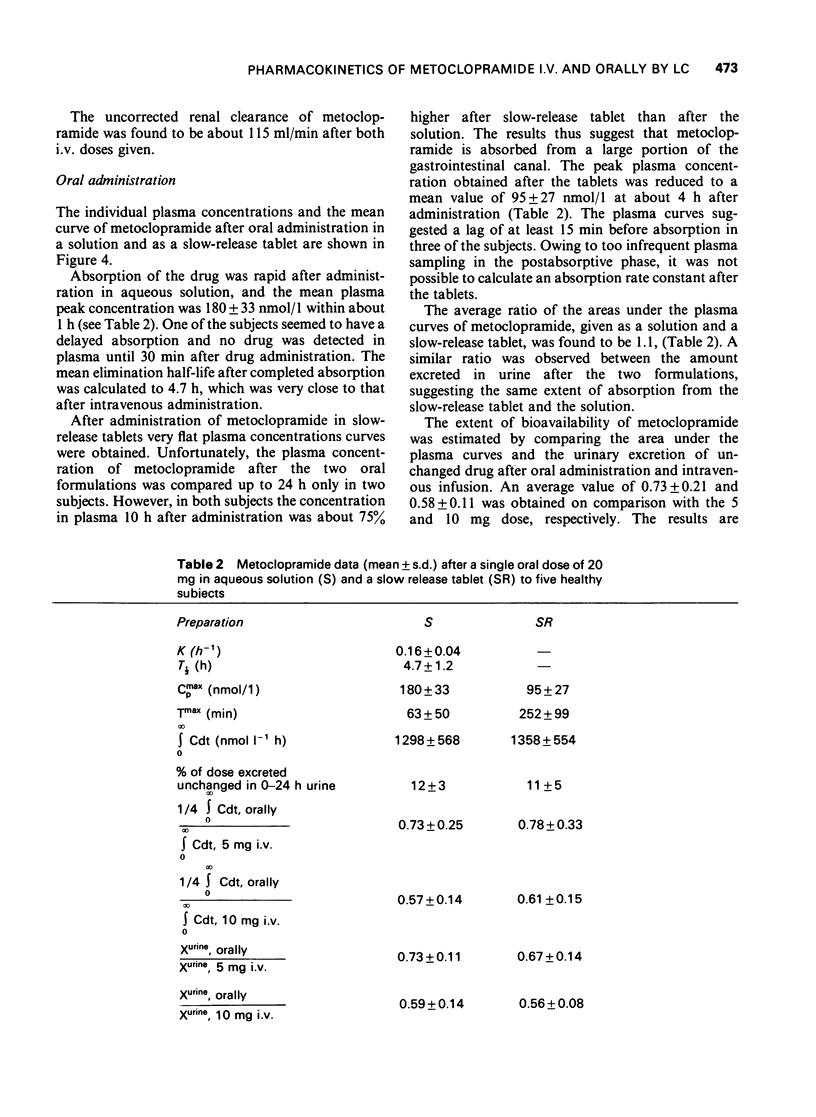
1 A rapid and sensitive method, based on liquid chromatography, has been developed for determination of metoclopramide concentrations in plasma and urine samples. Concentrations down to 15 nmol/1 (5 ng/ml) of plasma and 100 nmol/1 (30 ng/ml) of urine could be determined with a relative standard deviation of less than or equal to 10%. The method was used to study disposition of metoclopramide in healthy volunteers following single doses intravenously and orally as aqueous solution and a slow release tablet. 2 The initial distribution after intravenous administration was very rapid. The elimination half-life postdistribution was 4.9 h. The apparent volume of distribution, Vd, was 3.0 1/kg body weight. On average 19% was excreted unchanged after intravenous administration of 5 and 10 mg (15 and 30 mumol) of drug. The rate of absorption of metoclopramide was delayed after administration of a slow release tablet and the maximum plasma concentration was about 50% lower than after a solution. The extent of bioavailability was the same following the two different formulations suggesting a first-pass elimination of 25-40%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakke O. M., Segura J. The absorption and elimination of metoclopramide in three animal species. J Pharm Pharmacol. 1976 Jan;28(1):32–39. doi: 10.1111/j.2042-7158.1976.tb04019.x. [DOI] [PubMed] [Google Scholar]
- Bateman D. N., Davies D. S., Kahn C., Mashiter K. Pharmacokinetic and concentration-effect studies with metoclopramide [proceedings]. Br J Clin Pharmacol. 1977 Oct;4(5):650P–650P. doi: 10.1111/j.1365-2125.1977.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Bateman D. N., Davies D. S. Pharmacokinetics of metoclopramide. Lancet. 1979 Jan 20;1(8108):166–166. doi: 10.1016/s0140-6736(79)90568-3. [DOI] [PubMed] [Google Scholar]
- Bateman D. N., Kahn C., Mashiter K., Davies D. S. Pharmacokinetic and concentration-effect studies with intravenous metoclopramide. Br J Clin Pharmacol. 1978 Nov;6(5):401–407. doi: 10.1111/j.1365-2125.1978.tb04604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Morris D. W., Schoen H. J., DiMarino A. J. The effect of oral and intravenous metoclopramide on human lower esophageal sphincter pressure. Gastroenterology. 1976 Apr;70(4):484–487. [PubMed] [Google Scholar]
- Lavy S., Melamed E., Penchas S. Tardive dyskinesia associated with metoclopramide. Br Med J. 1978 Jan 14;1(6105):77–78. doi: 10.1136/bmj.1.6105.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Pinder R. M., Brogden R. N., Sawyer P. R., Speight T. M., Avery G. S. Metoclopramide: a review of its pharmacological properties and clinical use. Drugs. 1976;12(2):81–131. doi: 10.2165/00003495-197612020-00001. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Teng L., Bruce R. B., Dunning L. K. Metoclopramide metabolism and determination by high-pressure liquid chromatography. J Pharm Sci. 1977 Nov;66(11):1615–1618. doi: 10.1002/jps.2600661128. [DOI] [PubMed] [Google Scholar]


