Abstract
Androgen and its receptor (AR) have been reported to have pro- or antiapoptotic functions. However, the underlying molecular mechanism is incompletely understood. We report here that androgen and AR promote Bax-mediated apoptosis in prostate cancer cells. UV irradiation and ectopic expression of Bax induce apoptosis in AR-positive, but not AR-negative prostate cancer cells. UV- and Bax-induced apoptosis is abrogated in AR-positive cells that express small interference RNA (siRNA) of AR and is sensitized by reintroduction of AR into AR-negative cells. Although AR is able to promote Bax-mediated apoptosis independently of androgen, the promotion by AR can be further potentiated by androgen via AR-dependent transcription activation. AR is essential for the translocation of Bax to mitochondria in UV- or Bax-induced apoptosis. Inhibition of Bax expression by Bax siRNA suppresses UV-induced apoptosis in AR-positive cells. In addition, introduction of AR into AR-negative prostate cancer cells upregulates expression levels of the BH3-only protein Noxa, whereas inhibition of Noxa expression reduces the promotion by AR on UV-induced apoptosis. Thus, our results reveal a novel cross talk between the androgen/AR hormonal signaling pathway and the intrinsic apoptotic death pathway that determines the sensitivity of stress-induced apoptosis in prostate cancer cells.
The steroid hormone androgen is required for male sexual development and maintenance of the male phenotypes (38). In its target cells, androgen is converted to its reduced form by the specific 5-α-reductase (2, 5). The reduced form of androgen is active and exerts its biological functions via androgen receptor (AR) in androgen responsive tissues or organs (11, 47). AR is a member of the steroid hormone receptor superfamily and is a latent transcription factor (35, 48). In the absence of androgen, unliganded AR remains in the cytoplasm (21, 60). Upon binding to androgen, the androgen/AR complex translocates into the nucleus, where it induces expression of androgen response genes that are involved in many cellular activities, from proliferation to programmed cell death (2, 38, 60, 64). The activity of AR can also be regulated by protein phosphorylation, heat shock proteins, and dimerization (40, 62, 67).
The androgen/AR complex plays a critical role in the development of prostate cancer (7, 17, 18). The growth of prostate cancer is initially androgen dependent, and therefore androgen ablation has been a leading choice of metastatic prostate cancer therapy (30, 52). However, malignant prostate cancer eventually relapses and grows independently of androgen (16). An important feature of androgen-independent prostate cancer cells is that they are insensitive to apoptosis induced by hormonal therapy, conventional chemotherapy, and radiation treatment (28). Alternative strategies have been explored to induce apoptosis in androgen-independent prostate cancer cells. Previous studies have shown that downregulation of the antiapoptotic Bcl-2 family proteins such as Bcl-2 and Bcl-xL or upregulation of the proapoptotic Bcl-2 family proteins such as Bax can sensitize or trigger apoptosis in androgen-independent prostate cancer cells (22, 26).
The multidomain proapoptotic protein Bax plays a critical role in the intrinsic apoptotic pathway (1, 39, 58). In viable cells, Bax mainly exists as a monomer in the cytoplasm (29, 66). Upon stimulation by various death insults, Bax undergoes conformational changes and subsequently translocates to mitochondria, where it inserts into the outer membrane as oligomers, resulting in the release of cytochrome c and apoptosis (23, 51, 66). The proapoptotic activity of Bax is tightly controlled by many cellular regulators. Bcl-2 forms heterodimers with Bax and prevents its insertion into the mitochondrial membrane (3, 58). Therefore, the ratio of Bcl-2 to Bax is critical for the determination of the apoptotic threshold (71). On the other hand, the induction of the active conformation of Bax by death stimuli is mediated by the BH3-only proapoptotic Bcl-2 family proteins Bid, Bim, Noxa, and Puma and other yet-to-be identified proteins/lipids (9, 33, 41, 42, 56, 57). BH3-only subfamily members are known to induce apoptosis by association with antiapoptotic Bcl-2 family members or by stimulating other apoptosis-promoting factors (12, 41, 44, 57). Furthermore, the tumor suppressor and transcription factor p53 can enhance the proapoptotic activity of Bax, either inducing Bax expression or sequestrating Bcl-2 or Bcl-xL in the cytoplasm (4, 53). Many apoptotic stimuli, such as UV irradiation and anticancer agents, utilize Bax to kill cells (10, 72). Overexpression of Bax alone is also sufficient to induce apoptosis in many types of cells, including cultured androgen-independent prostate cancer cells, and to reduce prostate tumor size in the host animals as well (26, 46, 68, 69).
Androgen is known to suppress apoptosis in androgen-dependent prostate cancer cells via AR-mediated repression of apoptotic genes (19). However, the role of androgen in apoptosis of androgen-independent prostate cancer cells is controversial, being suggested to be proapoptotic or antiapoptotic (25, 31, 50, 61). Considering that in vivo a low amount of androgen is still produced even after castration, a paradox is whether androgen is beneficial or detrimental to the treatment of malignant prostate cancer. We show here that androgen and AR promote stress-mediated apoptosis via augmentation of BAX translocation to mitochondria and upregulation of Noxa protein expression.
MATERIALS AND METHODS
Reagents.
Antibodies against Bax (N20 and P19), Bak, AR (C19), and hemagglutinin (HA) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Bim antibody was from Stressgen (Victoria, British Columbia, Canada). Anti-PUMA antibody was from Calbiochem (La Jolla, CA). Anti-Noxa antibody was a generous gift from Y. Tesfaigiz, University of New Mexico. Antibody against Cox-IV (cytochrome c oxidase complex IV) was from Molecular Probes (Eugene, OR), and antibody against β-actin was from Sigma (St. Louis, MO). Polyclonal antibody against AR (AN-21) was described previously (36). Antibodies against Bcl-2, Bad, and Bid were generous gifts from Stanley Korsmeyer. The synthetic androgen R1881 was from New England Nuclear (Boston, MA), and the specific androgen antagonist Casodex (Cdx) was from AstraZeneca Pharmaceuticals (Wilmington, DE). A Super-Signal chemiluminescence detection kit was from Pierce Chemical Co. (Rockford, IL). The fluorogenic caspase-3 substrate DEVD-AFC (carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin) was purchased from Calbiochem (La Jolla, CA).
Cell culture and stable cell lines.
Human prostate cancer cell LNCaP sublines 104-S, 104-R1, and CDXR were generated and maintained as described previously (36). Androgen-dependent 104-S cells were grown in Dulbecco modified Eagle medium with 10% fetal bovine serum, supplemented with 1 nM DHT (5α-dihydrotestosterone). Androgen-independent 104-R1, CDXR, and PC3 cells were cultured in Dulbecco modified Eagle medium with 10% dextran-coated, charcoal-stripped fetal bovine serum (27).
Recombinant adenovirus infection and immunoblotting analysis.
The replication-deficient recombinant adenoviral vectors encoding Cre/LoxP inducible HA-Bax (Ad/Bax), Cre recombinase (Ad/Cre), or luciferase (Ad/Luc) were generated as described previously (69, 70). All recombinant adenoviruses were propagated in HEK293 cells, purified, and titrated by standard protocols (59). For viral infection, the ratio of Ad/Bax to Ad/Cre was 5:1 at a total multiplicity of infection of 50 −100 (70). In control experiments, Ad/Luc (luciferase) plus Ad/Cre or Ad/Bax plus Ad/GFP (green fluorescent protein) were kept at the same ratio.
Cell death and apoptosis assays.
Cell viability was measured by using a colorimetric method with 3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), according to the manufacturer's instructions (Promega, Madison, WI). For apoptotic death assays, cells were stained with Hoechst stain (H33258), visualized by UV microscopy, and quantitated by counting condensed and fragmented nuclei in five randomly selected areas unless described otherwise. The apoptosis-mediated alteration of membrane phospholipids was monitored by annexin V-fluorescein isothiocyanate and quantified by a fluorescence-activated cell sorter (FACS). Mitochondrial membrane potential (Δψm) was measured with 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (3), followed by FACS analysis (68). Caspase activity assays were carried out by using the fluorogenic caspase-3 substrates (DEVE-AFC) as described previously (68).
Subcellular fractionation.
LNCaP 104-R1 cells (107) were harvested by gently scraping cells off the dishes in culture medium, pelleted by centrifugation, and washed once with phosphate-buffered saline. Cells were resuspended in five times the volume of buffer A (20 mM HEPES [pH 7.4], 10 mM KCl, 1.5 mM MgCl2, 250 mM sucrose, 1 mM EGTA, 1 mM EDTA, and a cocktail of protease inhibitors). After incubation on ice for 30 min, cells were fragmented by passing through a 22-gauge needle 20 times (55). Cell homogenates were centrifuged at 900 × g for 5 min to remove unbroken cells and nuclei. The postnuclear homogenates were centrifuged at 16,000 × g for 30 min at 4°C to obtain the cytosolic fraction. The pellets were washed once with buffer A and then centrifuged at 16,000 × g for 30 min to obtain the mitochondrion-rich membrane fraction. The quality of each fraction was determined by immunoblotting with the antibody against the cytosolic marker β-actin, or the mitochondrial marker Cox IV (15).
RNA interference.
Complementary 64-base short-hairpin RNA oligonucleotides were synthesized (Integrated DNA Technologies, Inc., Coralville, IA) containing a 19-base C-terminal AR sequence (5′-GCACTGCTACTCTTCAGCA-3′) in inverted repeat orientation (6). After annealing, the 64-mer duplex was inserted into BglII/HindIII-digested pH1RP RNA interference expression vector (20). After transfection of LNCaP 104-R1 cells using Effectene reagent (QIAGEN, Valencia, CA), G418-resistant colonies were selected and clones were screened for reduced AR expression by immunoblotting analysis with anti-AR polyclonal antibody (AN21) (37). All siRNA duplexes were purchased from Dharmacon Research. The siRNA targeting sequence for Bax is 5′-GACGAACUGGACAGUAACA-3′, that for for Noxa is 5′-GGAAGUCGAGUGUGCUACU-3′, and that for luciferase is 5′-CGUACGCGGAAUACUUCGA-3′. The transfection of siRNA oligonucleotides was performed with Lipofectamine 2000 (Stratagene) according to the manufacturer's recommendations.
Site-directed mutagenesis.
AR mutation that substitutes tyrosine for a cysteine at amino acid 619 (C619Y) was created by using PCR-based site mutagenesis kit (QuikChange) from Stratagene according to the manufacturer's procedure. The forward mutation primer is 5′-CTTGTCGTCTTCGGAAATATTATGAAGCAGGGATGAC-3′, and the reverse mutation primer is 5′-GTCATCCCTGCTTCATAATATTTCCGAAGACGACAAG-3′. All PCR products and the mutation were verified by DNA sequences. Wild-type AR and the AR (C619Y) mutant were subcloned into pSG5 expression vector (Stratagene).
RESULTS
Ectopic expression of Bax and UV irradiation selectively induce apoptosis in AR-positive, but not AR-negative prostate cancer cells.
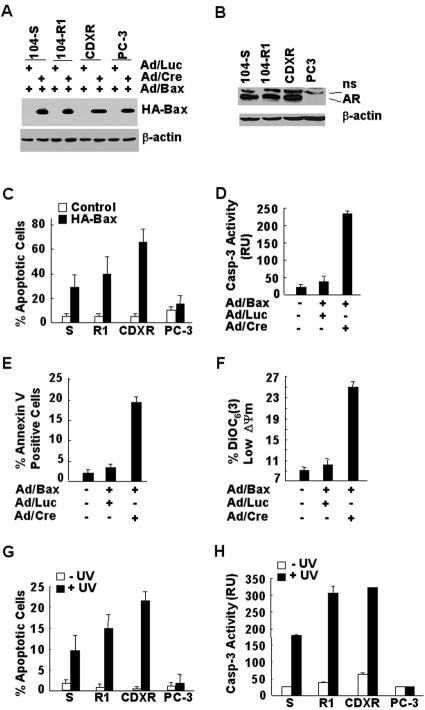
The proapoptotic Bcl-2 family protein Bax plays a critical role in apoptosis in many cell types (1, 24). Although Bax mediates apoptosis induced by many death signals, such as UV irradiation (10, 13), ectopic expression of Bax alone is also able to induce cell death (68). To understand how Bax-mediated apoptosis is regulated in androgen-independent prostate cancer cells, we used a Cre/loxP-inducible adenoviral vector encoding HA-Bax (Ad/Bax) (70). Coinfection of the cells with Ad/Bax, along with an adenoviral vector encoding bacterial P1 Cre recombinase (Ad/Cre) that specifically recognizes the loxP sites (32) results in expression of HA-Bax (69, 70). Cells of various prostate cancer cell lines were infected with Ad/Bax, along with Ad/Cre, or a control adenoviral vector encoding luciferase (Ad/Luc). Immunoblotting analysis with anti-HA antibody revealed that expression levels of HA-Bax were similar among different prostate cancer cells 24 h postinfection (Fig. 1A). The expression levels of HA-Bax were ∼2-fold greater than the endogenous Bax levels (data not shown). Among the cells examined, androgen-dependent LNCaP 104-S cells and androgen-independent LNCaP 104-R1 and CDXR cells were AR positive, whereas androgen-independent PC3 cells were AR negative (Fig. 1B). Although LNCaP 104-S, 104-R1, and CDXR cells were sensitive to Bax-induced cell death, PC3 cells were resistant (Fig. 1C). Bax-induced cell death had apoptotic features in 104-R1 cells, including caspase activation as measured by caspase activity assays (Fig. 1D), translocation of membrane phospholipid phosphatidylserine as detected by annexin V assays (Fig. 1E) and decreased mitochondrial potential as monitored by DiOC6(3) using FACS analysis (Fig. 1F). Similar results were obtained with other AR-positive prostate cancer cells examined (data not shown). Thus, Bax selectively induces apoptosis in AR-positive prostate cancer cells.
FIG. 1.
Bax and UV selectively induce apoptosis in AR-positive, but not in AR-negative prostate cancer cells. (A) Prostate cancer cell lines were infected with adenoviral vector encoding HA-Bax (Ad/Bax), Cre (Ad/Cre), or luciferase (Ad/Luc) (multiplicity of infection = 100, 24 h), as indicated. Expression of HA-Bax was analyzed by immunoblotting with anti-HA antibody. (B) Expression levels of AR in various prostate cancer cell lines in panel A were examined by immunoblotting with anti-AR antibody (AN21). (C) The viability of the infected cells in panel A was measured by MTS assays. S, 104-S; R1, 104-R1. (D, E, and F) Apoptotic cell death of the infected 104-R1 cells in panel A was characterized by caspase activity assays with the fluorogenic caspase-3 substrate DEVD-AFC (D), by detecting apoptotic membrane alteration with FITC-annexin V staining (E), and by measuring the mitochondrial membrane potential changes (F), as indicated. (G and H) The same panel of cells in panel A was treated with or without UV radiation (10 mJ/cm2) for 16 h. Apoptotic cells were detected and quantitated by nuclear staining with Hoechst (H33258) (G) or by caspase assays using the fluorogenic substrate DEVD-AFC (H). S, 104-S; R1, 104-R1.
To determine whether UV also selectively induces apoptosis in AR-positive cells, the above prostate cancer cells were exposed to UV (10 mJ/cm2, 16 h). The apoptotic cell death assays revealed that AR-positive 104-S, 104-R, and CDXR cells were sensitive to UV-induced apoptosis, whereas AR-negative PC3 cells were insensitive (Fig. 1G). Similar results were obtained with caspase activity assays (Fig. 1H). These results suggest that like Bax, UV also selectively induces apoptosis in AR-positive, but not in AR-negative prostate cancer cells.
AR is required for Bax-mediated apoptosis.
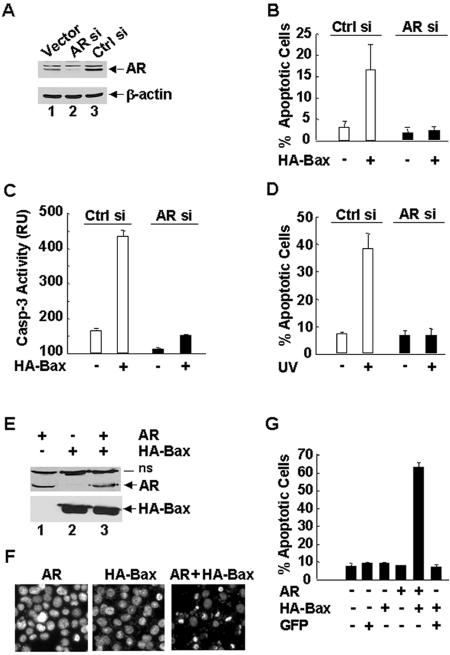
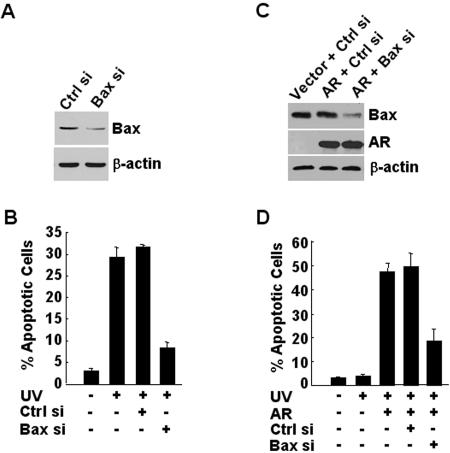
The above observations suggest that Bax-mediated apoptosis (ectopic expression of Bax- or UV-induced apoptosis) in prostate cancer cells may be regulated by AR. To test this hypothesis, we used 104-R1 cells that had been stably transfected with mammalian expression vectors encoding double-stranded siRNA for AR (AR siRNA) or the control scramble siRNA. Immunoblotting analysis with anti-AR antibody revealed that expression levels of AR proteins were dramatically decreased by AR siRNA but not by the control siRNA (Fig. 2A, compare lanes 2 and 3). As a control, the amount of β-actin was similar between AR knockdown and parental 104-R1 cells (Fig. 2A). Inhibition of AR expression by AR siRNA did not affect the cell growth rate or its androgen independency (data not shown). Ectopic expression of HA-Bax induced apoptosis in control 104-R1 cells, as measured by apoptotic cell death assays (Fig. 2B) and caspase activity assays (Fig. 2C). Under the same conditions, HA-Bax was unable to induce apoptosis in 104-R1 cells expressing AR siRNA (Fig. 2B and C). Similarly, apoptotic cell death induced by UV was abrogated in 104-R1 cells expressing AR siRNA. UV induced 40% apoptotic cell death in parental 104-R1 cells, but only 9% apoptotic cell death, which is similar to basal level of cell death, in 104-R1 cells expressing AR siRNA (Fig. 2D). These results suggest that AR is required for UV- and Bax-induced apoptosis in AR-positive prostate cancer cells.
FIG. 2.
AR is required for UV- and Bax-induced apoptosis in prostate cancer cells. (A) 104-R1 cells were stably transfected with mammalian expression vectors encoding AR siRNA (AR si), the control scramble siRNA (Ctrl si), or empty vector. Expression levels of AR proteins were examined by immunoblotting with anti-AR antibody (AN21). (B and C) 104-R1 (AR si and Ctrl si) cells were infected with Ad/Bax plus Ad/Cre (HA-Bax +) or Ad/Bax plus Ad/Luc (HA-Bax −) for 24 h. The apoptotic cell death was then detected and quantitated by Hoechst (H33258) staining (B) or by caspase activity assays with the fluorogenic substrate DEVD-AFC (C). (D) Cells were treated with UV (10 mJ/cm2) for 18 h. The apoptotic cell death was detected as described in panel B. (E, F, and G) AR-negative PC3 cells were transiently transfected with an expression vector encoding AR or green fluorescent protein (GFP). After 20 h, cells were infected with Ad/Bax plus Ad/Cre for another 16 h. (E) Expression of AR and HA-Bax proteins was analyzed by immunoblotting with antibodies against AR or HA, respectively. (F) The apoptotic cells were examined by nuclear staining with Hoechst (H33258) and visualized under UV fluorescence microscope. (G) Apoptotic cells were also quantitated by counting immunostaining AR-positive cells, which also have apoptotic nuclei as shown by staining with Hoechst stain (H33258). GFP-positive cells were quantitated in the same manner.
If AR affects the sensitivity of prostate cancer cells to Bax-mediated apoptosis, introduction of AR into AR-negative PC3 cells, which originally were resistant to Bax-induced apoptosis (Fig. 1C), should sensitize the cells to Bax killing. To test this notion, PC3 cells were transfected with mammalian expression vectors encoding AR or empty vector, followed by infection with Ad/HA-Bax, along with Ad/Cre or the control Ad/Luc. Immunoblotting analysis showed that AR-transfected PC3 cells expressed AR proteins, whose expression levels were similar in the presence or absence of HA-Bax (Fig. 2E). AR-expressing PC3 cells became sensitive to Bax-mediated apoptosis compared to control PC3 cells, as monitored by immunostaining AR+ cells that also have apoptotic nuclei as detected by Hoechst (H33258) staining (Fig. 2F and G). These data suggest that introduction of AR restores the sensitivity of AR-negative PC3 cells to Bax-mediated apoptosis.
Androgen, via AR-dependent transcription, potentiates Bax-mediated apoptosis.
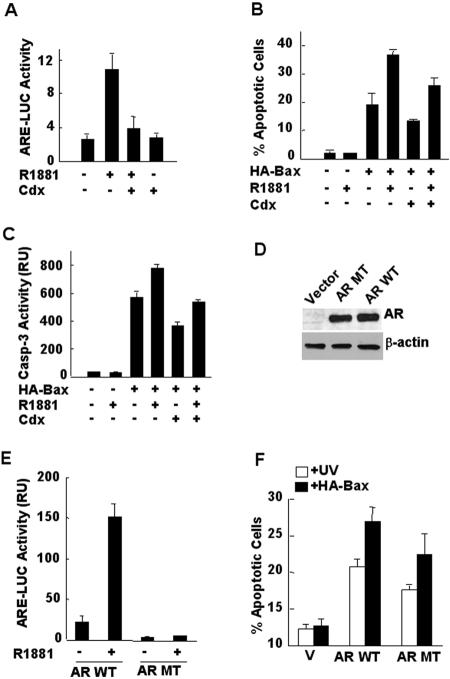
Typically, AR functions in a complex with androgen (38), although it has been reported that AR has androgen-independent functions under certain circumstances (36). The requirement of AR for apoptosis induced by ectopic expression of Bax or UV irradiation in prostate cancer cells prompted us to ask whether Bax-mediated apoptosis can be further regulated by androgen. To test this idea, we used a specific androgen inhibitor Casodex (Cdx), which significantly inhibited the stimulation by a synthetic androgen R1881 on the activity of ARE-LUC reporter gene in 104-R1 cells (Fig. 3A) (62, 63). 104-R1 cells were infected with Ad/HA-Bax, along with Ad/Cre or the control Ad/Luc, followed by treatment with or without R1881, in the presence or absence of Cdx. Apoptotic cell death assays revealed that R1881 potentiated Bax-mediated apoptosis (Fig. 3B, 37% cell death induced by HA-Bax plus R1881 in comparison to 19% cell death induced by HA-Bax alone). In the presence of Cdx, HA-Bax plus R1881 only induced 27% cell death (Fig. 3B). Consistently, Cdx abrogated the stimulatory effect of R1881 on HA-Bax-induced caspase-3 activation (Fig. 3C). Thus, the promotion of Bax-mediated apoptosis by androgen depends on its ability to stimulate the transcriptional activity of AR.
FIG. 3.
Androgen potentiates Bax-mediated apoptosis in 104-R1 cells via AR-dependent transcription. (A) AR transcriptional assays with ARE-luciferase. 104-R1 cells were transfected with ARE-luciferase reporter gene in the presence or absence of R1881 (1 nM). The specific androgen antagonist casodex (Cdx, 5 μM) was added to the culture medium 30 min prior to the addition of R1881 to block the transcription activity of AR. The activity of ARE-LUC was measured as described previously (54). (B) 104-R1 cells were infected with Ad/Bax, along with Ad/Cre or the control Ad/Luc, for 2 h. Infected cells were incubated with or without the synthetic androgen R1881 (1 nM) for another 15 h. Apoptotic death was measured by Hoechst (H33258) staining. (C) Caspase-3 activity assays with DEVD-AFC. (D) AR-negative PC3 cells were transfected with mammalian expression vectors encoding wild-type AR (AR WT), the transcription-deficient AR(C619Y) mutant (AR MT), or empty vector. Expression of AR proteins was detected by immunoblotting with anti-AR antibody (C19). β-Actin was used as a control. (E) PC3 cells were transfected with mammalian expression vectors encoding WT AR or AR(C619Y) mutant, along with ARE-luciferase reporter gene in the presence or absence of R1881 (1 nM). The activity of ARE-LUC was measured as described previously (54). (F) PC3 cells transfected with WT AR or AR(C619Y) mutant were irradiated with or without UV irradiation (10 mJ/cm2) or infected with Ad/HA-Bax, along with Ad/Cre or the control Ad/Luc. Apoptotic cells were visualized and quantitated by nuclear staining with Hoechst (H33258).
To directly determine whether the transcriptional activity of AR is involved in the promotion of Bax-mediated cell death by the androgen/AR complex, we used a transcriptional inactive AR(C619Y) mutant, in which cysteine 619 has been replaced by tyrosine that disrupts the coordination of zinc finger in AR DNA-binding domain and thereby eliminates AR DNA binding and transcription activity (54). AR-negative PC3 cells were transfected with mammalian expression vectors encoding wild-type (WT) AR and the AR(C619Y) mutant, along with ARE-LUC reporter gene, followed by stimulation with or without R1881. Immunoblotting analysis showed that WT AR and AR(C619Y) had similar levels of expression (Fig. 3D). As expected, expression of WT AR mediated R1881-induced ARE-LUC activity (Fig. 3E). In contrast, the AR(C619Y) mutant was unable to mediate R1881-induced ARE-LUC activity (Fig. 3E). Apoptotic cell death assays revealed that PC3 cells expressing the transcriptionally inactive AR(C619Y) mutant had less apoptosis induced by ectopic expression of HA-Bax- or UV compared to PC3 cells expressing WT AR (Fig. 3F). These results suggest that although AR itself can promote Bax-mediated apoptosis, androgen-stimulated AR transcription activity was also involved in sensitizing cells to Bax- and UV-mediated cell death.
The translocation of Bax to mitochondria requires AR and can be enhanced by androgen.
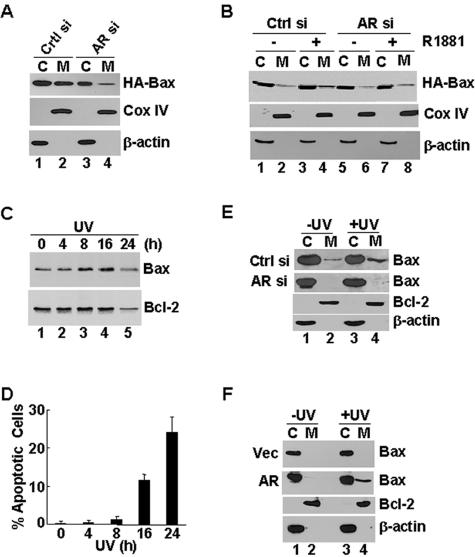
For Bax to kill, it needs to undergo conformational changes, oligomerize, and translocate to the outer membrane of mitochondria (65, 66). To understand how Bax-mediated apoptosis is regulated by androgen and AR, we determined the effects of androgen and AR on the translocation of Bax to mitochondria. 104-R1 cells stably expressing AR siRNA or control siRNA were infected with Ad/HA-Bax, along with Ad/Cre or Ad/Luc. Cellular fractionation in combination with immunoblotting analysis revealed that under the condition of androgen deprivation, there was significant HA-Bax in both cytoplasmic and mitochondrion-rich fractions in control 104-R1 cells 24 h postinfection (Fig. 4A, compare lane 1 and lane 2). This is consistent with the observations that under the same conditions 104-R1 cells underwent apoptosis (Fig. 2B). In contrast, when AR expression was inhibited by AR siRNA (Fig. 2A), the distribution of HA-Bax to mitochondria-rich fractions was significantly reduced (Fig. 4A, compare lane 4 to lane 2). This is consistent with the observations that under the same conditions 104-R1(AR siRNA) cells were resistant to HA-Bax-induced apoptosis (Fig. 2B). The difference of HA-Bax distribution between cytoplasmic and mitochondrion-rich fractions was not the result of incomplete separation of cytoplasmic and mitochondrion fractions, as monitored by immunoblotting analysis of the cytoplasmic marker β-actin and the mitochondrial marker COX IV (Fig. 4B). We also determined whether Bax translocation to mitochondria could be further regulated by androgen. At an earlier time point (16 h postinfection), the distribution of HA-Bax to mitochondrion-rich fractions was clearly potentiated by androgen in control 104-R1 cells (Fig. 4B, compare lane 4 to lane 2). The augmentation by androgen on the distribution of HA-Bax to mitochondrion-rich fractions depended on AR since it was abolished by inhibition of AR expression by siRNA (Fig. 4B, compare lane 8 to lane 4). Thus, AR is required for Bax translocation to mitochondria, which can be further augmented by androgen.
FIG. 4.
Positive regulation of Bax translocation to mitochondria by AR and the androgen/AR complex. (A) 104-R1 cells stably transfected with AR siRNA (AR si) or the control siRNA (Ctrl si) were infected with Ad/Bax, along with Ad/Cre or the control Ad/Luc, under the conditions of androgen deprivation. After 24 h, cells were fractionated into the cytosolic fraction and the mitochondrion-rich membrane fraction by differential centrifugation. (A) The localization of HA-Bax was examined by immunoblotting with anti-HA antibody. The separation of the cytoplasm from mitochondria was monitored by immunoblotting with the antibody against β-actin, which is a cytosolic marker, and the antibody against Cox IV, which is a mitochondrial marker. (B) Control (Ctrl si) or AR knockdown (AR si) 104-R1 cells were infected as described in panel A in the presence or absence of R1881 (1 nM). The localization of HA-Bax was analyzed as described in panel A, except the infection time was only 16 h. (C and D) 104-R1 cells were irradiated with or without UV (10 mJ/cm2) for various times as indicated. A set of sample aliquots was used to determine the translocation of endogenous Bax to mitochondrion-rich fractions (C), and another set of the aliquots was used to analyze the progression of apoptosis (D). (E and F) Control (Ctrl si) and AR knockdown (AR si) 104-R1 cells (E) and PC3 cells transfected with expression vector encoding AR or empty vector (F) were irradiated with or without UV (10 mJ/cm2) for 16 h and then fractionated (C, cytosol; M, membrane). The fractions were examined with antibodies against Bax. Bcl-2 and β-actin were used as controls (only control immunoblots of 104-R1 [Ctrl si] and PC3 [AR] are shown).
Next, we examined the effect of AR on the translocation of endogenous Bax to mitochondria in response to UV irradiation. In 104-R1 cells, the translocation of endogenous Bax to mitochondrion-rich membrane fractions reached near maximum by 8 h after UV irradiation (Fig. 4C), at which time there was no significant apoptosis (Fig. 4D). Thus, during this period of time, UV-induced translocation of Bax to mitochondria was prior to apoptosis, rather than the consequence of cell death. Under these conditions, the translocation of Bax induced by UV irradiation was inhibited in 104-R1 cells expressing AR siRNA compared to that in 104-R1 cells expressing the control siRNA (Fig. 4E). Conversely, UV only induced the translocation of Bax to mitochondria in AR-expressing PC3 cells but not in AR-negative PC3 cells (Fig. 4F). In contrast, the distribution of Bcl-2 between cytoplasmic and mitochondrion-rich membrane fractions was not affected by AR (Fig. 4E and F), suggesting that AR promotes the translocation of Bax to mitochondria independently of Bcl-2. Taken together, AR promotes Bax-mediated apoptosis via regulation of Bax translocation to mitochondria.
Bax is an effector of AR in UV-induced apoptosis.
The requirement of AR for the mitochondria translocation of Bax prompted us to test whether Bax is involved in the promotion by AR on apoptosis. To test this possibility, AR-positive 104-R1 cells expressing Bax siRNA, which specifically inhibited Bax expression (Fig. 5A), or the control siRNA, were exposed UV. Apoptotic cell death assays revealed that UV-induced apoptosis was significantly reduced in 104-R1 cells expressing Bax siRNA but not in 104-R1 cells expressing the control siRNA (Fig. 5B). Thus, Bax was indeed involved in UV-induced apoptosis in AR-positive 104-R1 cells. Similar results were obtained with AR-expressing PC3 cells. Bax expression was significantly inhibited by its siRNA (Fig. 5C), but not the control siRNA, in AR-expressing PC3 cells. Apoptotic cell death assays revealed that UV induced apoptosis in AR-expressing PC3 cells but not in AR-negative PC3 cells (Fig. 5D, compare lane 2 to lane 3). The apoptosis induced by UV in AR-expressing PC3 cells was significantly inhibited by Bax siRNA but not by the control siRNA (Fig. 5D, compare lane 4 to lane 5). Taken together, Bax mediates, at least in part, the promotion of AR on UV-induced apoptosis in AR-positive cells.
FIG. 5.
Bax is required for UV-induced apoptosis in AR-positive cells. (A and B) AR-positive 104-R1 cells were transfected with Bax siRNA (Bax si) or its control siRNA (Ctrl si). After 20 h, cells were irradiated with or without UV (10 mJ/cm2) for 16 h. Expression of Bax was analyzed by immunoblotting with anti-Bax antibody (A), and the apoptotic cell death was monitored by Hoechst (H33258) staining (B). (C and D) AR-negative PC3 cells were transfected with mammalian expression vector encoding AR or empty vector, along with Bax siRNA or the control siRNA for 18 h before irradiation with or without UV (10 mJ/cm2). Expressions of Bax and AR were analyzed by immunoblotting with antibody against Bax or AR, respectively, in panel C. The apoptotic cell death were visualized and quantitated by nuclear staining with Hoechst stain (H33258) in panel D.
The BH3-only protein Noxa is also involved in the promotion by AR on Bax-mediated cell death.
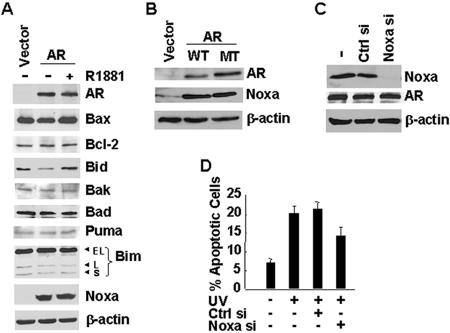
The proapoptotic activity of Bax is negatively regulated by the antiapoptotic Bcl-2 family proteins Bcl-2 but positively regulated by the proapoptotic Bcl-2 family BH3-only proteins such as Bad and Bik (44). It is possible that the androgen/AR complex promotes apoptosis induced by ectopic expression of Bax and UV irradiation via regulation of the Bcl-2 network. To test this scenario, AR-negative PC3 cells were transfected with an expression vector encoding AR or an empty vector, followed by treatment with or without R1881. Immunoblotting analysis revealed that among eight Bcl-2 family proteins examined, including Bax, Bcl-2, Bid, Bak, Bad, Puma, Bim, and Noxa, only the expression of the BH3-only protein Noxa was significantly induced by AR (Fig. 6A, compare lane 2 to lane 1). However, R1881 did not further stimulate AR-induced expression of Noxa (Fig. 6A, compare lane 3 to lane 2). Expression of the transcription-deficient AR(C619Y) mutant at the levels similar to that of WT AR also induced expression of Noxa in PC3 cells (Fig. 6B). These results suggest that the induction of Noxa by AR was independent on its transcription activity.
FIG. 6.
Noxa is involved in the promotion by AR on Bax-mediated apoptosis. (A) AR-negative PC3 cells were transfected with expression vector encoding AR or empty vector in the presence or absence of R1881 (1 nM). After 24 h, cells were harvested and subjected to immunoblotting analysis with antibodies against AR (C19), Bax, Bcl-2, Bid, Bak, Bad, Puma, Bim, and Noxa. (B) PC3 cells were transfected with expression vectors encoding WT AR or the AR(C619Y) mutant or empty vector for 24 h. Cell lysates were subjected to immunoblotting analysis with antibodies against AR and Noxa. (C and D) PC3 cells were cotransfected with AR, along with Noxa siRNA or control siRNA, for 24 h, followed by UV irradiation (10 mJ/cm2) for an additional 14 h. Cell lysates were subjected to immunoblotting analysis with antibodies against Noxa and AR (C); the apoptotic cell death was visualized and quantitated by nuclear staining with Hoechst stain (H33258) (D).
Noxa is a BH3-only protein which is involved in apoptosis via activation of the intrinsic apoptotic pathway (57). The upregulation of Noxa protein expression by AR may contribute to its promotion of cell death. To test this hypothesis, PC3 cells were transfected with expression vector encoding AR or empty vector, along with Noxa siRNA or the control siRNA. AR induced Noxa expression was not affected by cotransfected control siRNA but significantly inhibited by Noxa siRNA (Fig. 6C). The inhibitory effect of Noxa siRNA was specific since the expression of AR or β-actin was not affected (Fig. 6C). To determine whether upregulation of Noxa by AR contributes to Bax-mediated cell death, PC3 cells expressing AR, along with Noxa siRNA or control siRNA, were irradiated with UV. Apoptotic cell death assays revealed that expression of AR sensitized cell to UV-induced apoptosis (Fig. 6D). The promotion of UV-induced apoptosis by AR was partially inhibited by Noxa siRNA but not the control siRNA (Fig. 6D). These data suggest that the promotion by AR on Bax-mediated cell death was partially mediated by its upregulation of Noxa expression.
DISCUSSION
The multidomain proapoptotic Bcl-2 family protein Bax plays a critical role in the intrinsic apoptotic pathway (1, 39, 58, 65). Overexpression of Bax alone is sufficient to induce apoptosis (68), and Bax also mediates the apoptotic signal from many death stimuli such as UV irradiation (10, 65, 72). Although it has been shown that the proapoptotic activity of Bax can be inhibited by Bcl-2 and be promoted by some of the BH3-only proteins or other yet-to-be identified proteins/lipids (9, 33, 41, 42, 56), the underlying mechanism is incompletely understood. We demonstrate here that androgen and AR promote stress-mediated apoptosis via augmentation of BAX translocation to mitochondria and upregulation of Noxa protein expression. This conclusion is based on the following evidence.
First, AR-positive 104-R1, 104-S, and CDXR cells, but not AR-negative PC3 cells, were sensitive to apoptosis induced by UV irradiation and ectopic expression of Bax (Fig. 1). Second, inhibition of AR expression by AR siRNA in AR-positive 104-R1 cells abolished UV- and Bax-induced apoptosis (Fig. 2), whereas introduction of AR into AR-negative PC3 cells sensitized the cells to these apoptotic insults (Fig. 2). Third, AR promoted Bax-mediated apoptosis via transcription-dependent and -independent manners (Fig. 3). Fourth, AR and the androgen/AR complex were able to promote the translocation of Bax to mitochondria (Fig. 4). Fifth, Bax is a major downstream effector of AR in promoting UV-induced apoptosis in AR-positive prostate cancer cells (Fig. 5). Sixth, the promotion by AR on Bax-mediated apoptosis was partially mediated by its upregulation of the BH3-only protein Noxa protein levels (Fig. 6). Taken together, these results demonstrate that androgen and AR positively regulate the proapoptotic activity of Bax in prostate cancer cells.
The role of the male steroid hormone androgen in apoptosis of androgen-independent prostate cancer cells is highly controversial, being suggested to have an antiapoptotic or proapoptotic role (25, 31, 50, 61). It has been reported that androgen induces apoptosis in AR-expressing PC3 cells (25). In contrast, it has been shown that the androgen antagonist Cdx (62, 63) induces apoptosis in AR-positive LNCaP cells, suggesting that androgen may be required for cell survival (43). Consistent with this, it has been reported that androgen blocks apoptosis induced by tumor necrosis factor alpha or FasL in LNCaP cells (34). Our results demonstrated that androgen promotes Bax-mediated apoptosis (Fig. 3 and 4) in AR-positive prostate cancer cells. Therefore, androgen may exert different effects on apoptosis depending upon the cell context and the death stimulus.
How does androgen promote Bax-mediated apoptosis? Our results showed that the promotion by androgen depended on AR transcription activity. Under androgen-deprivation conditions, AR was required for UV- and HA-Bax-induced apoptosis (Fig. 2B, 2C, 3B, and 3C). Addition of androgen further promoted Bax-mediated apoptosis in 104-R1 cells (Fig. 3B and C). However, this promotion was abolished by the androgen agonist Cdx, which inhibited the transcription activity of AR (Fig. 3A). In the presence of Cdx, the promotion of Bax killing by androgen/AR was reduced to the levels similar to that seen in the absence of androgen (Fig. 3B and C). Thus, the stimulation by androgen on Bax-mediated apoptosis depended on the transcription activity of AR. This notion was further supported by the observations that the transcription-deficient AR(C619Y) mutant (Fig. 3E) was less potent than WT AR in promoting Bax-mediated apoptosis (Fig. 3F). How androgen-mediated transcription promotes Bax killing has yet to be determined. Androgen treatment did not induce detectable changes in expression of known Bax regulators, including antiapoptotic Bcl-2 family members such as Bcl-2 and proapoptotic Bcl-2 family proteins such as Bid, Bad, Bak, Puma, and Noxa (Fig. 6A). Detailed mechanisms apart, our results showed that androgen via AR was able to promote the translocation of Bax to mitochondria in the early phase of HA-Bax-induced apoptosis (Fig. 4C), thereby enhancing apoptosis (Fig. 4D). Future studies are needed to determine which androgen target gene(s) mediates the promotion by androgen on Bax-mediated apoptosis.
AR is essential for Bax-mediated apoptosis in AR-positive prostate cancer cells. Normally, AR functions as a transcription factor after binding to its ligand androgen (38). However, AR also has androgen-independent functions (36). Our results showed that under androgen-deprivation conditions, inhibition of AR expression by AR siRNA (Fig. 2A) abrogated UV- and Bax-induced apoptosis in 104-R1 cells (Fig. 2B, C, and D), whereas introduction of AR into AR-negative PC3 cells (Fig. 2E) sensitized the cells to Bax-mediated apoptosis (Fig. 2F and G). In addition, blockade of AR transcriptional activity by Cdx only inhibited the promotion by androgen but not by AR on Bax-mediated apoptosis (Fig. 3A, B, and C). This suggests that nongenotropic AR activity may be involved in this regulation. This conclusion is further supported by the finding that the transcription-deficient AR(C619Y) mutant (Fig. 3E), although it was less potent than WT AR, was still able to promote UV- and Bax-induced apoptosis (Fig. 3F). These findings put AR in the same group as Nur77/TR3 and other steroid hormone receptors that have ligand-independent functions (8, 45). It has been reported that Nur77/TR3, which is an orphan member of the steroid-thyroid hormone nuclear receptor family, can bind to Bcl-2 and convert the latter from a protector to a killer, thereby activating the intrinsic apoptotic pathway (8, 49). Note that the requirement for AR in Bax-mediated apoptosis may be affected by other cellular signaling regulators. We found that AR-negative androgen-independent DU145 cells, which are deficient of retinoblastoma protein, were less sensitive to Bax killing than 104-R1 cells, but nevertheless they were less resistant than PC3 cells (data not shown).
How does AR promote apoptosis? The translocation of Bax to mitochondria is one of the key steps for Bax-mediated apoptosis (23, 66). Our results showed that AR was required for UV to induce the translocation of endogenous Bax to mitochondria (Fig. 4C), prior to apoptosis (Fig. 4D). Inhibition of AR expression by AR siRNA also suppressed the translocation of exogenous HA-Bax (Fig. 4A), thereby inhibiting HA-Bax-induced apoptosis (Fig. 2B and C). The translocated Bax did insert into the outer membrane of mitochondria since it was resistant to alkali treatment (data not shown). Thus, our results provide a mechanistic link between the androgen/AR pathway and the proapoptotic protein Bax. It is still incompletely understood how AR promotes the translocation of Bax to mitochondria. There was no detectable translocation of AR to mitochondria, nor is there evidence of direct interaction between AR and Bax (data not shown). Our results also showed that AR was able to upregulate the protein levels of the BH3-only Noxa independently of its transcription activity (Fig. 6A and B). Inhibition of AR-induced upregulation of Noxa proteins (Fig. 6C) partially suppressed UV-induced apoptosis (Fig. 6D), suggesting that the upregulation of Noxa by AR is involved in the promotion by AR on apoptosis. It has been reported that murine Noxa selectively interacts with antiapoptotic Bcl-2 family protein Mcl-1, and in coordination with other BH3-only proteins, to promote apoptosis (12, 57). Unlike murine Noxa, which has two BH3 domains, human Noxa only has a single BH3 domain, and the exact mechanism(s) by which human Noxa induces apoptosis has yet to be determined. It is possible that Noxa may coordinate with Bax in the promotion of AR on stress-induced apoptosis. Future studies are needed to test this hypothesis.
The finding that Bax-mediated apoptosis requires AR and can be further enhanced by androgen in AR-positive androgen-independent prostate cancer cells has potentially important implications in prostate cancer therapy. After castration, prostate cancer will eventually grow in an androgen-independent manner, and recent studies have shown that administration of androgen to castrated mice can suppress the growth of androgen-independent tumors in vivo (14). Our results provided a molecular mechanism by which androgen suppresses growth of androgen-independent tumors and demonstrate that potentially therapeutic interventions via Bax-mediated apoptosis may be more suitable for AR-positive, but not AR-negative prostate cancer.
Acknowledgments
This study was partially supported by National Institutes of Health grant CA090516 (to J.X.).
Footnotes
This study is dedicated in memory of Jialing Xiang's mentor, Stanley Korsmeyer, who passed away due to lung cancer on 31 March 2005.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. M., and S. Liao. 1968. Selective retention of dihydrotestosterone by prostatic nuclei. Nature 219:277-279. [DOI] [PubMed] [Google Scholar]
- 3.Antonsson, B., F. Conti, A. Ciavatta, S. Montessuit, S. Lewis, I. Martinou, L. Bernasconi, A. Bernard, J. J. Mermod, G. Mazzei, K. Maundrell, F. Gambale, R. Sadoul, and J. C. Martinou. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science 277:370-372. [DOI] [PubMed] [Google Scholar]
- 4.Baptiste, N., and C. Prives. 2004. p53 in the cytoplasm: a question of overkill? Cell 116:487-489. [DOI] [PubMed] [Google Scholar]
- 5.Bruchovsky, N., and J. D. Wilson. 1968. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 243:2012-2021. [PubMed] [Google Scholar]
- 6.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, G., R. A. Irvine, G. A. Coetzee, and W. D. Tilley. 2001. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 20:207-223. [DOI] [PubMed] [Google Scholar]
- 8.Cao, X., W. Liu, F. Lin, H. Li, S. K. Kolluri, B. Lin, Y. H. Han, M. I. Dawson, and X. K. Zhang. 2004. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol. Cell. Biol. 24:9705-9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartron, P. F., T. Gallenne, G. Bougras, F. Gautier, F. Manero, P. Vusio, K. Meflah, F. M. Vallette, and P. Juin. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell 16:807-818. [DOI] [PubMed] [Google Scholar]
- 10.Cartron, P. F., P. Juin, L. Oliver, S. Martin, K. Meflah, and F. M. Vallette. 2003. Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Mol. Cell. Biol. 23:4701-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, C., J. Kokontis, S. Swift, and S. T. Liao. 1990. Molecular cloning and structural analysis of complementary DNA of human and rat androgen receptors. Prog. Clin. Biol. Res. 322:53-63. [PubMed] [Google Scholar]
- 12.Chen, L., S. N. Willis, A. Wei, B. J. Smith, J. I. Fletcher, M. G. Hinds, P. M. Colman, C. L. Day, J. M. Adams, and D. C. Huang. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17:393-403. [DOI] [PubMed] [Google Scholar]
- 13.Cho, S., S. L. O'Connor, and T. J. McDonnell. 2002. Evidence that nucleotide excision repair is attenuated in bax-deficient mammalian cells following ultraviolet irradiation. Exp. Cell Res. 278:158-165. [DOI] [PubMed] [Google Scholar]
- 14.Chuu, C. P., R. A. Hiipakka, J. Fukuchi, J. M. Kokontis, and S. Liao. 2005. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 65:2082-2084. [DOI] [PubMed] [Google Scholar]
- 15.Cotteret, S., Z. M. Jaffer, A. Beeser, and J. Chernoff. 2003. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol. Cell. Biol. 23:5526-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford, E. D., M. Rosenblum, A. M. Ziada, and P. H. Lange. 1999. Hormone refractory prostate cancer. Urology 54:1-7. [DOI] [PubMed] [Google Scholar]
- 17.Culig, Z. 2003. Role of the androgen receptor axis in prostate cancer. Urology 62:21-26. [DOI] [PubMed] [Google Scholar]
- 18.Debes, J. D., and D. J. Tindall. 2002. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett. 187:1-7. [DOI] [PubMed] [Google Scholar]
- 19.Denmeade, S. R., X. S. Lin, and J. T. Isaacs. 1996. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 28:251-265. [DOI] [PubMed] [Google Scholar]
- 20.Fukuchi, J., R. A. Hiipakka, J. M. Kokontis, S. Hsu, A. L. Ko, M. L. Fitzgerald, and S. Liao. 2004. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 64:7682-7685. [DOI] [PubMed] [Google Scholar]
- 21.Georget, V., J. M. Lobaccaro, B. Terouanne, P. Mangeat, J. C. Nicolas, and C. Sultan. 1997. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol. Cell Endocrinol. 129:17-26. [DOI] [PubMed] [Google Scholar]
- 22.Gleave, M. E., H. Miayake, J. Goldie, C. Nelson, and A. Tolcher. 1999. Targeting bcl-2 gene to delay androgen-independent progression and enhance chemosensitivity in prostate cancer using antisense bcl-2 oligodeoxynucleotides. Urology 54:36-46. [DOI] [PubMed] [Google Scholar]
- 23.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross, A., J. M. McDonnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 25.Heisler, L. E., A. Evangelou, A. M. Lew, J. Trachtenberg, H. P. Elsholtz, and T. J. Brown. 1997. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol. Cell Endocrinol. 126:59-73. [DOI] [PubMed] [Google Scholar]
- 26.Honda, T., B. T. Gjertsen, K. B. Spurgers, F. Briones, S. J. Lee, M. L. Hobbs, R. E. Meyn, J. A. Roth, C. Logothetis, and T. J. McDonnell. 2001. Restoration of bax in prostate cancer suppresses tumor growth and augments therapeutic cell death induction. Anticancer Res. 21:3141-3146. [PubMed] [Google Scholar]
- 27.Horwitz, K. B., Y. Koseki, and W. L. McGuire. 1978. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology 103:1742-1751. [DOI] [PubMed] [Google Scholar]
- 28.Howell, S. B. 2000. Resistance to apoptosis in prostate cancer cells. Mol. Urol. 4:225-231. [PubMed] [Google Scholar]
- 29.Hsu, Y. T., K. G. Wolter, and R. J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94:3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huggins, C., and C. V. Hodges. 1941. Studies on prostatic cancer. The effect of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1:293-397. [DOI] [PubMed] [Google Scholar]
- 31.Joly-Pharaboz, M. O., A. Ruffion, A. Roch, L. Michel-Calemard, J. Andre, J. Chantepie, B. Nicolas, and G. Panaye. 2000. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J. Steroid Biochem. Mol. Biol. 73:237-249. [DOI] [PubMed] [Google Scholar]
- 32.Kanegae, Y., K. Takamori, Y. Sato, G. Lee, M. Nakai, and I. Saito. 1996. Efficient gene activation system on mammalian cell chromosomes using recombinant adenovirus producing Cre recombinase. Gene 181:207-212. [DOI] [PubMed] [Google Scholar]
- 33.Kim, T. H., Y. Zhao, M. J. Barber, D. K. Kuharsky, and X. M. Yin. 2000. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J. Biol. Chem. 275:39474-39481. [DOI] [PubMed] [Google Scholar]
- 34.Kimura, K., M. Markowski, C. Bowen, and E. P. Gelmann. 2001. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer Res. 61:5611-5618. [PubMed] [Google Scholar]
- 35.Kokontis, J., S. Liao, and C. Chang. 1991. Transcriptional activation by TR3 receptor, a member of the steroid receptor superfamily. Receptor 1:261-270. [PubMed] [Google Scholar]
- 36.Kokontis, J., K. Takakura, N. Hay, and S. Liao. 1994. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 54:1566-1573. [PubMed] [Google Scholar]
- 37.Kokontis, J. M., N. Hay, and S. Liao. 1998. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol. Endocrinol. 12:941-953. [DOI] [PubMed] [Google Scholar]
- 38.Kokontis, J. M., and S. Liao. 1999. Molecular action of androgen in the normal and neoplastic prostate. Vitam. Horm. 55:219-307. [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer, S. J.1999. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 59:1693s-1700s. [PubMed] [Google Scholar]
- 40.Kuiper, G. G., and A. O. Brinkmann. 1995. Phosphotryptic peptide analysis of the human androgen receptor: detection of a hormone-induced phosphopeptide. Biochemistry 34:1851-1857. [DOI] [PubMed] [Google Scholar]
- 41.Kuwana, T., L. Bouchier-Hayes, J. E. Chipuk, C. Bonzon, B. A. Sullivan, D. R. Green, and D. D. Newmeyer. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17:525-535. [DOI] [PubMed] [Google Scholar]
- 42.Kuwana, T., M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green, and D. D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111:331-342. [DOI] [PubMed] [Google Scholar]
- 43.Lee, E. C., P. Zhan, R. Schallhom, K. Packman, and M. Tenniswood. 2003. Antiandrogen-induced cell death in LNCaP human prostate cancer cells. Cell Death Differ. 10:761-771. [DOI] [PubMed] [Google Scholar]
- 44.Letai, A., M. C. Bassik, L. D. Walensky, M. D. Sorcinelli, S. Weiler, and S. J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183-192. [DOI] [PubMed] [Google Scholar]
- 45.Li, H., S. K. Kolluri, J. Gu, M. I. Dawson, X. Cao, P. D. Hobbs, B. Lin, G. Chen, J. Lu, F. Lin, Z. Xie, J. A. Fontana, J. C. Reed, and X. Zhang. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159-1164. [DOI] [PubMed] [Google Scholar]
- 46.Li, X., M. Marani, J. Yu, B. Nan, J. A. Roth, S. Kagawa, B. Fang, L. Denner, and M. Marcelli. 2001. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 61:186-191. [PubMed] [Google Scholar]
- 47.Liao, S. 1994. Androgen action: molecular mechanism and medical application. J. Formos. Med. Assoc. 93:741-751. [PubMed] [Google Scholar]
- 48.Liao, S. S., J. Kokontis, T. Sai, and R. A. Hiipakka. 1989. Androgen receptors: structures, mutations, antibodies and cellular dynamics. J. Steroid Biochem. 34:41-51. [DOI] [PubMed] [Google Scholar]
- 49.Lin, B., S. K. Kolluri, F. Lin, W. Liu, Y. H. Han, X. Cao, M. I. Dawson, J. C. Reed, and X. K. Zhang. 2004. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527-540. [DOI] [PubMed] [Google Scholar]
- 50.Linja, M. J., K. J. Savinainen, O. R. Saramaki, T. L. Tammela, R. L. Vessella, and T. Visakorpi. 2001. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 61:3550-3555. [PubMed] [Google Scholar]
- 51.Marzo, I., C. Brenner, N. Zamzami, J. M. Jurgensmeier, S. A. Susin, H. L. Vieira, M. C. Prevost, Z. Xie, S. Matsuyama, J. C. Reed, and G. Kroemer. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281:2027-2031. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto, H., E. M. Messing, and C. Chang. 2004. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate 61:332-353. [DOI] [PubMed] [Google Scholar]
- 53.Miyashita, T., and J. C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293-299. [DOI] [PubMed] [Google Scholar]
- 54.Nazareth, L. V., D. L. Stenoien, W. E. Bingman III, A. J. James, C. Wu, Y. Zhang, D. P. Edwards, M. Mancini, M. Marcelli, D. J. Lamb, and N. L. Weigel. 1999. A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1). Mol. Endocrinol. 13:2065-2075. [DOI] [PubMed] [Google Scholar]
- 55.Nijhawan, D., M. Fang, E. Traer, Q. Zhong, W. Gao, F. Du, and X. Wang. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oakes, S. A., L. Scorrano, J. T. Opferman, M. C. Bassik, M. Nishino, T. Pozzan, and S. J. Korsmeyer. 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 102:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053-1058. [DOI] [PubMed] [Google Scholar]
- 58.Oltvai, Z. N., C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- 59.Rosenfeld, M. E., M. Feng, S. I. Michael, G. P. Siegal, R. D. Alvarez, and D. T. Curiel. 1995. Adenoviral-mediated delivery of the herpes simplex virus thymidine kinase gene selectively sensitizes human ovarian carcinoma cells to ganciclovir. Clin. Cancer Res. 1:1571-1580. [PubMed] [Google Scholar]
- 60.Tomura, A., K. Goto, H. Morinaga, M. Nomura, T. Okabe, T. Yanase, R. Takayanagi, and H. Nawata. 2001. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J. Biol. Chem. 276:28395-28401. [DOI] [PubMed] [Google Scholar]
- 61.Umekita, Y., R. A. Hiipakka, J. M. Kokontis, and S. Liao. 1996. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc. Natl. Acad. Sci. USA 93:11802-11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veldscholte, J., C. A. Berrevoets, A. O. Brinkmann, J. A. Grootegoed, and E. Mulder. 1992. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry 31:2393-2399. [DOI] [PubMed] [Google Scholar]
- 63.Waller, A. S., R. M. Sharrard, P. Berthon, and N. J. Maitland. 2000. Androgen receptor localization and turnover in human prostate epithelium treated with the antiandrogen, casodex. J. Mol. Endocrinol. 24:339-351. [DOI] [PubMed] [Google Scholar]
- 64.Wang, G., E. Reed, and Q. Q. Li. 2004. Apoptosis in prostate cancer: progressive and therapeutic implications. Int. J. Mol. Med. 14:23-34. [PubMed] [Google Scholar]
- 65.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong, C. I., Z. X. Zhou, M. Sar, and E. M. Wilson. 1993. Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J. Biol. Chem. 268:19004-19012. [PubMed] [Google Scholar]
- 68.Xiang, J., D. T. Chao, and S. J. Korsmeyer. 1996. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93:14559-14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiang, J., J. Gomez-Navarro, W. Arafat, B. Liu, S. D. Barker, R. D. Alvarez, G. P. Siegal, and D. T. Curiel. 2000. Pro-apoptotic treatment with an adenovirus encoding Bax enhances the effect of chemotherapy in ovarian cancer. J. Gene Med. 2:97-106. [DOI] [PubMed] [Google Scholar]
- 70.Xiang, J., A. Piche, C. Rancourt, J. Gomez-Navarro, G. Siegal, R. Alvarez, and D. T. Curiel. 1999. Ad inducible recombinant adenoviral vector encoding Bax selectively induces apoptosis in ovarian cancer cells. Tumor Targeting 4:1-9. [Google Scholar]
- 71.Yin, X. M., Z. N. Oltvai, and S. J. Korsmeyer. 1994. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369:321-323. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]