Abstract
Gametocytes, the sexual stages of malaria parasites (Plasmodium spp.) that are transmissible to mosquitoes, have been the focus of much recent research as potential targets for novel drug and vaccine therapies. However, little is known about the host clearance of gametocyte-infected erythrocytes (GEs). Using a number of experimental strategies, we found that the scavenger receptor CD36 mediates the uptake of nonopsonized erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum by monocytes and culture-derived macrophages (Mφs). Light microscopy and immunofluorescence assays revealed that stage I and IIA gametocytes were readily internalized by monocytes and Mφs. Pretreating monocytes and Mφs with a monoclonal antibody that blocked CD36 resulted in a significant reduction in phagocytosis, as did treating GEs with low concentrations of trypsin to remove P. falciparum erythrocyte membrane protein 1 (PfEMP-1), a parasite ligand for CD36. Pretreating monocytes and Mφs with peroxisome proliferator-activated receptor γ-retinoid X receptor agonists, which specifically upregulate CD36, resulted in a significant increase in the phagocytosis of GEs. Murine CD36 on mouse Mφs also mediated the phagocytosis of P. falciparum stage I and IIA gametocytes, as determined by receptor blockade with anti-murine CD36 monoclonal antibodies and the lack of uptake by CD36-null Mφs. These results indicate that phagocytosis of stage I and IIA gametocytes by monocytes and Mφs appears to be mediated to a large extent by the interaction of PfEMP-1 and CD36, suggesting that CD36 may play a role in innate clearance of these early sexual stages.
Species of the protozoan genus Plasmodium are intraerythrocytic parasites that are the causative agents of malaria. Each year, there are 300 million to 500 million cases of malaria and 1.5 million to 2.7 million attributable fatalities (3). Many of these deaths occur in children and are the result of severe and cerebral malaria caused by Plasmodium falciparum, the most pathogenic of the four species that infect humans.
P. falciparum is unique among human malaria species in that erythrocytes infected with this parasite are believed to evade clearance by immune cells of the spleen by sequestering in the microvasculature of various tissues and organs, including the skin, lung, gut, muscle, heart, and brain (30). Sequestration is mediated by cytoadherence of parasitized erythrocytes (PEs) to microvascular endothelial cells (reviewed in reference 19). Trophozoites and schizonts of P. falciparum express ligands, including P. falciparum erythrocyte membrane protein 1 (PfEMP-1) (6, 7), on the surface of PEs. These ligands enable cytoadherence of PEs to various endothelial cell receptors, including the leukocyte differentiation antigen CD36 (32, 34, 35), intercellular adhesion molecule 1 (ICAM-1) (9, 33), thrombospondin (TSP) (36), integrin αvβ3 (42), chondroitin sulfate (16), and hyaluronic acid (8).
The scavenger receptor CD36, an 88-kDa integral membrane protein that is recognized by most natural isolates of P. falciparum as a major sequestration receptor (31, 33), has been implicated in the pathogenesis of severe malaria. However, since little CD36 is expressed on cerebral microvascular endothelial cells (1, 51), it is more likely that other receptors, including perhaps ICAM-1 that is upregulated by inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) (29), are responsible for the binding of PEs in the microvasculature of the brain. CD36 is also expressed on monocytes and monocyte-derived macrophages (Mφs), phagocytic cells that are involved in the innate immune response and represent the first line of defense against malaria parasites. Recently, McGilvray and colleagues (28) described a novel mechanism of nonopsonic phagocytosis of trophozoites and schizonts of P. falciparum by monocytes and culture-derived Mφs. Internalization of PEs was found to be mediated by an interaction between parasite ligands, including PfEMP-1, and CD36. This nonopsonic phagocytic mechanism may represent an important first line of defense against falciparum malaria in nonimmune individuals in which antibody-mediated opsonic uptake is expected to be less.
Treatment of monocytes and Mφs with agonists of the peroxisome proliferator-activated receptor γ (PPARγ)-retinoid X receptor (RXR) complex upregulates CD36 expression in these cells (48). Recently, incubation of monocytes and Mφs with PPARγ-RXR agonists, including 15d-Δ12,14-prostaglandin J2 (15d-PGJ2), 9-cis-retinoic acid (9-cis-RA), and the thiazolidinedione class of drugs (25), was shown to increase the internalization of erythrocytes containing asexual stages of P. falciparum (40). This increase in phagocytosis of PEs was accompanied by a decrease in parasite-induced TNF-α production. These results indicate that specific upregulation of Mφ CD36 by these compounds may represent a novel means for modulating host clearance of PEs and proinflammatory responses to P. falciparum.
P. falciparum undergoes an indeterminate number of cycles of asexual intraerythrocytic schizogony during an infection. After each cycle, a proportion of merozoites invade erythrocytes and differentiate into gametocytes, the sexual stages of the parasite (5). Mature male and female gametocytes undergo gametogenesis, fertilization, and sporogonic development in the midguts of mosquitoes of the genus Anopheles after these insects take a blood meal from an infected human. Gametocytes develop through five stages of gametocytogenesis from merozoite invasion of erythrocytes to elongated mature forms, a process that takes 8 to 10 days. A recent focus of research has involved the investigation of sexual differentiation of malaria parasites and the characterization of gametocyte proteins in order to determine potential targets for drugs and vaccines (24).
Mature stage V gametocytes circulate freely in the bloodstream, but stage I to IV gametocytes sequester in the microvasculature of various organs (37). Hayward and colleagues (22) reported that PfEMP-1 is the primary ligand responsible for binding of stage I and IIA gametocytes to CD36 expressed on C32 cells and that the mechanism of this cytoadherence is indistinguishable from that of asexual parasites. Rogers and colleagues (37) showed that later gametocytes (stages III and IV) cytoadhere to human bone marrow cell lines and that a PfEMP-1-CD36 interaction is not involved in this binding.
Based on these observations, we hypothesized that erythrocytes containing stage I and IIA gametocytes of P. falciparum (GEs) may be internalized by human monocytes and Mφs via nonopsonic phagocytosis mediated by CD36. While not of direct benefit to the host, such a mechanism might represent a potential means of clearing these early transmission stages from a nonimmune host. In addition, we wished to test whether upregulation of CD36 after treatment with PPARγ-RXR agonists resulted in increased clearance of gametocytes. Such a strategy in a clinical setting may represent a novel means for decreasing transmission of malaria parasites.
MATERIALS AND METHODS
Media and reagents.
Endotoxin-free RPMI 1640 culture medium, heat-inactivated fetal bovine serum, and heat-inactivated group AB human serum were obtained from Wisent (Mississauga, Canada). Dextran T500, Ficoll-Paque, and Percoll were obtained from Pharmacia (Peapack, N.J.). Triton X-100 was obtained from BDH (Toronto, Ontario, Canada), and 15d-PGJ2 was obtained from BIOMOL (Plymouth, Pa.). Sorbitol, HEPES, sodium bicarbonate, hypoxanthine, 0.9% saline, sterile water, trypsin-EDTA, dimethyl sulfoxide, 9-cis-RA, bovine serum albumin (BSA), propidium iodide, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG), 4′,6-diamidino-2-phenylindole (DAPI), and paraformaldehyde were obtained from Sigma-Aldrich (Oakville, Canada). Mouse anti-human monoclonal immunoglobulins (IgG1) used in phagocytosis assays included anti-CD36 clone FA6-152 (Beckman Coulter, Mississauga, Canada), anti-ICAM-1 clone 15.2 (Santa Cruz Biotech, Balthesa, Calif.), anti-αvβ3 clone 23C6 (Serotec, Raleigh, N.C.), anti-TSP clone C6.7 (Neomarkers, Union City, Calif.), and anti-CD45 clone T29 (Dako, Carpentaria, Calif.). Mouse anti-human monoclonal immunoglobulin (IgG1) clone HIT8a recognizes human CD8a and was obtained from eBioscience (San Diego, Calif.). Monoclonal anti-mouse CD36 IgA (clone 63) was generated from hybridomas of spleen cells taken from CD36-null mice after intravenous injection of recombinant adenovirus containing full-length murine CD36 cDNA (the adenovirus was a kind gift from Fred deBeer, University of Kentucky). Rat anti-mouse monoclonal immunoglobulin (IgG1) clone 30-F11 recognizes murine CD45 and was obtained from BD Pharmigen (Mississauga, Ontario, Canada). Human IgG Fc fragments were obtained from Calbiochem (San Diego, Calif.), and mouse IgG Fc fragments were obtained from Jackson Immunoresearch Laboratories (West Grove, Pa.). Mouse anti-parasite monoclonal immunoglobulin (IgG1) 93A3 (11), kindly provided by David Baker and Geoffrey Targett, targets Pfs16, a P. falciparum sexual stage-specific parasitophorous vacuole membrane protein that can be first be detected in gametocytes 35 h after merozoites invade erythrocytes (4, 12).
Cultures.
P. falciparum clone 3D7 (53) was cultured in vitro by using standard procedures (49) and modifications outlined by Smith et al. (46). The methods of Carter and colleagues (14) were used to induce and synchronize gametocytogenesis in culture and to differentiate the various stages of gametocytes. Trophozoites and schizonts, the only other stages that are taken up by monocytes and Mφs (23), were removed with an efficiency of 99% from gametocyte cultures by treating the cultures with 5% sorbitol at 150 and 30 min before phagocytosis assays were begun. The effectiveness of this purification method was evaluated by allowing gametocyte cultures to grow for 24 h after sorbitol treatment; in these cultures, rings were observed in very low numbers (maximum, 1% compared to gametocytes), indicating that very low numbers of asexual trophozoites were present in the gametocyte preparation. Cultures were washed three times in RPMI 1640 medium just prior to phagocytosis assays to remove free pigment. In experiments in which trypsinized GEs were used, cultures were treated with a 0.05% trypsin-EDTA solution, incubated at 37°C for 30 min, and washed twice in RPMI 1640 medium prior to phagocytosis assays.
Preparation of human monocytes and murine Mφs.
Monocytes were isolated from the venous blood of some of us (T.G.S., S.N.P., and K.C.K.) as described previously (28), by using a method that consistently yields a platelet-free population of nonactivated monocytes. About 2.5 × 105 purified human monocytes were adhered to circular glass coverslips (diameter, 18 mm) in 12-well culture plates. Monocytes, cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (RPMI-10) at 37°C in the presence of 5% CO2, were aged for 5 days to derive Mφs, which were assayed for phagocytic ability or surface levels of CD36.
Thioglycolate-derived Mφs were collected from the peritoneal cavities of both wild-type and CD36-null mice (20). These Mφs were plated directly onto circular glass coverslips in 12-well culture plates containing RPMI-10 and incubated at 37°C in the presence of 5% CO2.
Phagocytosis assays.
To study nonopsonic phagocytosis of GEs by human monocytes and Mφs in culture plates, Fc receptors were first blocked by incubating cells for 30 min with 20 μg of human IgG Fc fragments per ml. For receptor inhibition experiments, some wells containing monocytes and Mφs were also incubated with 10-μg/ml solutions of monoclonal antibodies to CD36, ICAM-1, integrin αvβ3, TSP, or CD45 (isotype control) or combinations of these antibodies. After the monocytes and Mφs were washed twice with RPMI-10, 500-μl portions of a P. falciparum culture containing only ring stages and various stages of gametocytes were layered on the monocytes and Mφs at a GE/phagocyte ratio of 2:1. The plates were rotated gently for 2 h at 37°C in the presence of 5% CO2. Coverslips containing monocytes and Mφs were then washed for 1 min in 4°C water to lyse parasitized erythrocytes that had bound to monocytes and Mφs but were not internalized. After the coverslips were fixed with methanol and stained with Giemsa stain, the phagocytic index (the percentage of 500 monocytes and Mφs that had completely internalized at least one GE) was determined for each coverslip. For the most part, the same protocol was used for studies of nonopsonic phagocytosis of GEs by murine Mφs. However, Fc receptors on murine Mφs were blocked by addition of 23 μg of murine IgG Fc fragments per ml, whereas CD36 was blocked by using a 1:10 dilution of anti-murine CD36. A concentration of anti-murine CD45 of 5 μg/ml was used as an isotype control for experiments with murine Mφs.
In experiments in which PPAR-RXRγ activation was examined, human Mφs were treated with RPMI-10 containing 5 μM 15d-PGJ2 and 1 μM 9-cis-RA or appropriate concentrations of dimethyl sulfoxide as a control and incubated at 37°C in the presence of 5% CO2 for 48 h.
Immunofluorescence assays.
After lysis of noninternalized GEs and uninfected erythrocytes, monocytes and Mφs on coverslips were fixed and permeabilized in acetone for 10 s. Smears of asexual and gametocyte cultures were also made on coverslips and fixed in the same manner. Coverslips were first washed with block buffer (0.1% Triton X-100 and 3% BSA in 1× phosphate-buffered saline [PBS]) for 30 min and then incubated with 10 μl of monoclonal antibody 93A3 (diluted 1:10 in block buffer) for 45 min. The coverslips were washed in block buffer for 5 min and then incubated with 10 μl of FITC-conjugated anti-mouse IgG (diluted 1:50 in block buffer) containing the nuclear fluorochrome propidium iodide (1 μg/ml) (for phagocytosis assays) or DAPI (1 μg/ml) (for culture smears) for 30 min. The coverslips were washed with wash buffer (0.1% Triton X-100 and 3% BSA in 1× PBS) for 5 min, and each coverslip was inverted onto a 10-μl drop of the anti-fade agent Vectashield that was applied to a glass slide and sealed at its edge with nail polish.
Detection of CD36 surface levels on Mφs.
Human Mφs, including controls and Mφs in which CD36 was upregulated, were assayed for surface levels of CD36. Cells gently scraped off wells of tissue culture dishes were stained with a 1:100 dilution (in 1% BSA in 1× PBS) of anti-CD36 monoclonal antibodies for 30 min, followed by a 1:50 dilution (in 1% BSA in 1× PBS) of a secondary FITC-conjugated anti-mouse IgG. The controls included secondary antibody-stained cells alone. Mφs were then fixed in 0.5% paraformaldehyde in PBS and analyzed by using an EPICS ELITE flow cytometer and software (Beckman-Coulter, Marseille, France)
Statistical analysis.
The data were expressed as means and standard deviations of n experiments. Statistical significance was determined by using the Student t test. Each mean represents the results of at least three equivalent and independent experiments, and each experiment was performed in duplicate. There was some variation between experiments due to the use of different monocyte donors; however, the results were consistent within each experiment. For Fig. 2 to 6, the phagocytic index values for coverslips incubated with various antibodies were normalized against controls (which were given a value of 100) for each experiment.
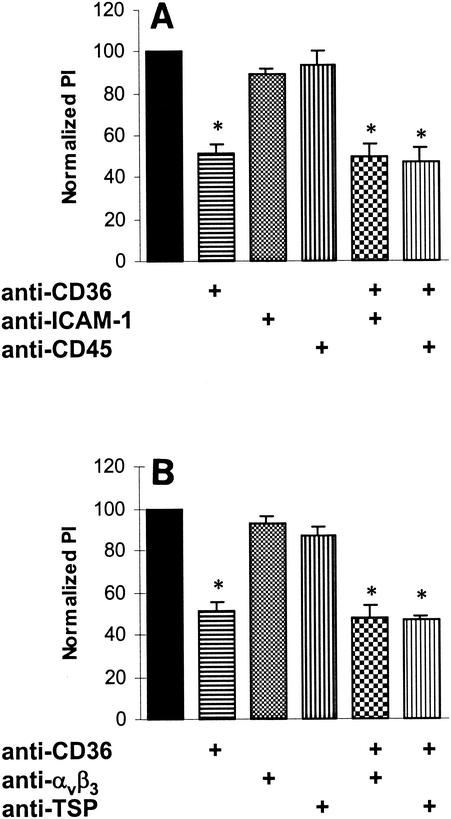
FIG. 2.
Anti-CD36 antibody FA6 reduces phagocytosis of stage I and IIA gametocytes by monocytes and Mφs. Prior to phagocytosis assays, monocytes or culture-derived Mφs (day 5) were incubated with a panel of monoclonal antibodies to various monocyte and Mφ surface receptors. Some of these receptors, including CD36 and ICAM-1, have been implicated in the cytoadherence of P. falciparum-infected erythrocytes via ligands on the surfaces of infected cells (see text), whereas CD36, integrin αvβ3, and TSP have been shown to be involved in the phagocytosis of apoptotic neutrophils (38). (A) Blocking CD36 on monocytes and Mφs with 10 μg of FA6 (anti-CD36 monoclonal antibody) per ml resulted in a significant decrease in phagocytosis of nonopsonized erythrocytes infected with stage I and IIA gametocytes (mean inhibition, 49.0%; standard deviation, 4.4%). An asterisk indicates that the P value is <0.01 (n = 5). (B) Antibodies (10 μg/ml) to ICAM-1 and to CD45 (shown in panel A) or to integrin αvβ3 and TSP (shown in panel B) did not result in a significant decrease in phagocytosis or had only a minor effect on phagocytosis (n = 3). Blocking with FA6 in combination with antibodies to the receptors ICAM-1, integrin αvβ3, TSP, and CD45 caused a reduction in phagocytosis that was similar to that observed with FA6 alone (n = 3). Significant reductions in phagocytosis (P < 0.01) are indicated by an asterisk. PI, phagocytic index.
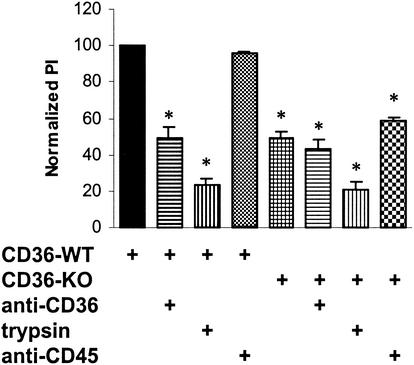
FIG. 6.
Murine CD36 mediates the uptake of stage I and IIA gametocytes by murine Mφs. Wild-type (WT) or CD36-null (KO) murine Mφs were incubated in the presence or absence of anti-murine CD36 monoclonal antibodies prior to phagocytosis assays with untreated or trypsin-treated cultures. Phagocytosis of GEs by wild-type murine Mφs was significantly reduced (mean ± standard deviation, 50.7% ± 5.6%) when it was blocked with anti-murine CD36 (P < 0.01; n = 3). Phagocytosis of GEs by CD36-null murine Mφs was reduced by 51.0% ± 4.0% compared to phagocytosis by wild-type murine Mφs (P < 0.01; n = 3), and treatment with anti-murine CD36 did not significantly reduce phagocytosis further. Treating cultures with 0.05% trypsin significantly reduced phagocytosis of GEs, by 77.1% ± 4.1% for wild-type murine Mφs (P < 0.01; n = 3) and by 79.6% ± 4.3% for CD36-null Mφs (P < 0.01; n = 3). Significant reductions in phagocytosis (P < 0.01) are indicated by an asterisk. PI, phagocytic index.
RESULTS
Phagocytosis of erythrocytes infected with gametocytes of P. falciparum.
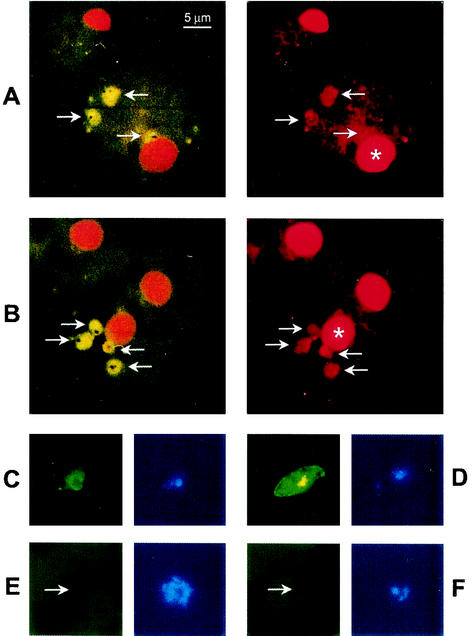
Erythrocytes containing stage I and IIA gametocytes of P. falciparum were internalized by monocytes and monocyte-derived Mφs in the presence of Fc receptor blockade and in the absence of both complement and antibodies to P. falciparum. FITC-labeled monoclonal antibody 93A3, which specifically recognizes gametocytes (4, 12), revealed that several GEs could be taken up by a single monocyte or Mφ (Fig. 1). This nonopsonic phagocytosis occurred under highly stringent conditions consisting of a ratio of GEs to monocytes or Mφs of 2:1, which was lower than the 20:1 ratio of asexual PEs to monocytes or Mφs that was used in previous studies of nonopsonic phagocytosis (28). Such stringency was designed to reflect the low numbers of gametocytes (compared to asexual parasites) present in natural P. falciparum infections (47).
FIG. 1.
Phagocytosis of GEs by Mφs. Cultures of nonopsonized erythrocytes infected with stage I and IIA gametocytes of P. falciparum were incubated for 2 h with Fc receptor-blocked human Mφs that were adhered to glass coverslips. After hypotonic lysis to remove noninternalized GEs, the monocytes on coverslips were fixed and prepared for monoclonal antibody immunofluorescence. Two pairs of fluorescence micrographs (A and B) show representative Mφs that contain internalized nonopsonized erythrocytes containing stage I and IIA gametocytes. Internalized gametocytes (arrows) fluoresce green due to the presence of FITC-conjugated monoclonal antibody 93A3 (a mouse IgG1), which targets the gametocyte surface protein Pfs16 on GEs (left micrographs in panels A and B). Nuclei of gametocytes and Mφs (asterisks) fluoresce orange due to the presence of propidium iodide, which targets DNA (right micrographs in panels A and B). FITC-conjugated 93A3 targets Pfs16 on various stages of gametocytes, including stage I (left micrograph in panel C) and stage III (right micrograph in panel D), in blood smears. When asexual parasites (one of which is indicated by an arrow) are incubated in blood smears with FITC-conjugated 93A3, they do not express Pfs16 (left micrograph in panel E). Gametocytes (one of which is indicated by an arrow) in blood smears do not react with FITC-conjugated monoclonal antibody HIT8a (a mouse IgG1 that targets human CD8a), which was used as an IgG1 isotype control (left micrograph in panel F). Nuclei of parasites fluoresce blue due to the presence of DAPI, which targets DNA (right micrographs in panels C to F). Bar = 5 μm.
Anti-CD36 antibodies reduce uptake of stage I and IIA gametocytes by monocytes and Mφs.
Prior to phagocytosis assays, monocytes or culture-derived Mφs (day 5) were incubated with a panel of monoclonal antibodies to various Mφ surface receptors that are involved in phagocytosis or are known to interact with PfEMP-1. For these experiments (Fig. 2) and the experiments whose results are shown in Fig. 3 to 5, typically 20 to 25% of the control monocytes and Mφs internalized at least one stage I or IIA GE. Figure 2 shows that CD36 receptor blockade of culture-derived Mφs with 10 μg of monoclonal antibody FA6 per ml caused a significant decrease (∼50%) in the phagocytosis of nonopsonized erythrocytes containing stage I and IIA gametocytes. Treatment of Mφs with 10 μg of monoclonal antibodies to other receptors (including ICAM-1, integrin αvβ3, TSP, and CD45) per ml did not result in a significant decrease in phagocytosis or had only a minor effect on phagocytosis. Phagocytosis of uninfected erythrocytes by monocytes or culture-derived Mφs was not observed. Thus, CD36 is a major receptor mediating the binding and phagocytosis of GEs by Mφs in vitro.
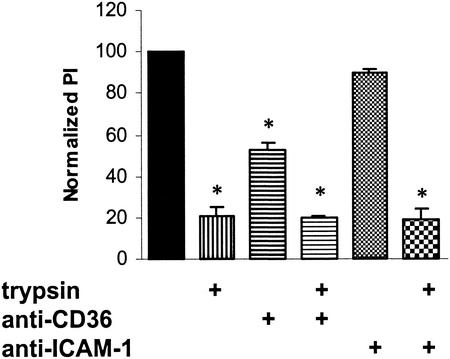
FIG. 3.
Treating parasitized erythrocytes with trypsin reduces phagocytosis of gametocytes by monocytes and Mφs. Cultures containing nonopsonized GEs were incubated with a low concentration of trypsin for 30 min and then washed repeatedly before they were allowed to interact with monocytes and Mφs. Phagocytosis of trypsin-treated GEs was reduced by 79.2% ± 4.5% (mean ± standard deviation) compared to phagocytosis of control GEs. Combining trypsin treatment and antibody blockade of monocytes and Mφs with 10 μg of either anti-CD36 or anti-ICAM-1 per ml did not result in a greater decrease in phagocytosis compared to the results obtained with trypsin alone. Significant reductions in phagocytosis (P < 0.01; n = 3) are indicated by an asterisk. PI, phagocytic index.
FIG. 5.
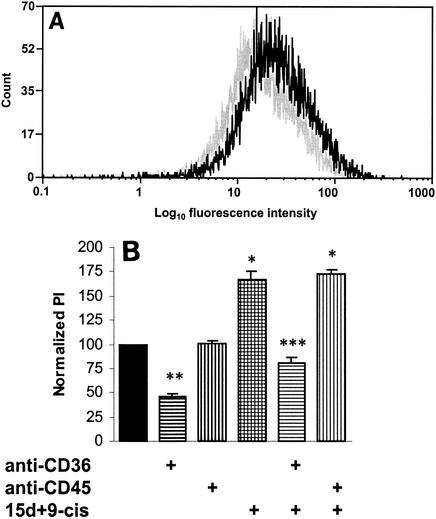
Upregulating CD36 increases phagocytosis of stage I and IIA gametocytes. (A) Human Mφs were incubated for 48 h with PPARγ-RXR agonists (5 μM 15d-PGJ2 and 1 μM 9-cis-RA) and processed for flow cytometry. In Mφs that were incubated with PPARγ-RXR agonists (black lines), there was an increase in CD36 surface levels compared to the levels in untreated Mφs (grey lines). (B) Phagocytosis assays were carried out by using adherent Mφs that had been incubated for 48 h with PPARγ-RXR agonists. Phagocytosis of nonopsonized erythrocytes containing stage I and IIA gametocytes was increased in Mφs treated with 5 μM 15d-PGJ2 and 1 μM 9-cis-RA (15d+9-cis) by 66.6% ± 9.4% (mean ± standard deviation) compared to phagocytosis in the controls (P < 0.01; n = 3) (one asterisk). Blocking CD36 with 10 μg of FA6 per ml in untreated Mφs resulted in a mean reduction in phagocytosis of 53.9% (P < 0.01; n = 3) (two asterisks), whereas anti-CD36 blockade of treated Mφs caused a mean reduction in phagocytosis of 52.0% (P < 0.01; n = 3) (three asterisks). PI, phagocytic index.
Treating GEs with trypsin reduced their uptake by monocytes and Mφs.
Trypsin has been shown to remove PfEMP-1, a ligand that mediates the binding of the parasite to CD36 (6). Parasite cultures were incubated with a low concentration of trypsin and then washed repeatedly before they were allowed to interact with monocytes and Mφs. Treating nonopsonized GEs with trypsin resulted in a ∼80% decrease in phagocytosis of stage I and IIA gametocytes compared to phagocytosis of control GEs (Fig. 3). Trypsin treatment in combination with antibody blockade of monocytes and Mφs with anti-CD36 or anti-ICAM-1 did not result in a greater decrease in phagocytosis than treatment with trypsin alone. These results indicate that one or more trypsin-sensitive ligands, most likely including PfEMP-1, mediate the binding and internalization of GEs by human monocytes and Mφs.
Phagocytosis of early- and late-stage gametocytes by monocytes and Mφs.
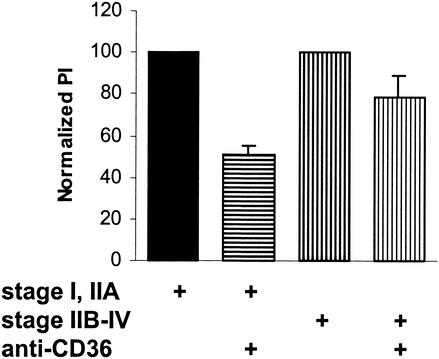
P. falciparum cultures were prepared so that at least 90% of all gametocytes were at stage I or IIA (early stages) or at least 90% of all gametocytes were at stages IIB to IV (late stages). In these assays, there was significantly greater uptake of early stages (∼20 to 25%) than of late stages (∼5 to 8%) (P < 0.01; n = 4). After incubation with 10 μg of anti-CD36 antibodies per ml for 30 min, monocytes and Mφs were incubated with early- or late-stage gametocyte cultures for 2 h. Figure 4 shows that blocking CD36 on monocytes and Mφs with FA6 reduced the phagocytosis of nonopsonized erythrocytes containing late-stage gametocytes by 21.2% (mean) compared to controls, which is significantly less that the 49.0% reduction in phagocytosis recorded with early-stage GEs (P < 0.05; n = 4). If the reduction in uptake of stage IIB to IV gametocytes had been due in part to the internalization of contaminating stage I and IIA gametocytes in the late-stage gametocyte cultures, then there would have been an even greater difference in uptake between the two types of gametocytes. These data indicate that CD36 is a primary receptor involved in the phagocytosis of early-stage GEs by monocytes and Mφs but plays a lesser role in the internalization of late-stage gametocytes.
FIG. 4.
Phagocytosis of early- and late-stage gametocytes by monocytes and Mφs. Cultures were prepared so that at least 90% of all gametocytes were at stage I or IIA (early stages) or so that at least 90% of all gametocytes were at stages IIB to IV (late stages). After incubation with 10 μg of anti-CD36 antibody FA6 per ml, monocytes and Mφs were incubated with early- or late-stage gametocyte cultures for 2 h. Blocking CD36 on monocytes and Mφs with FA6 reduced the phagocytosis of late-stage GEs by 21.2% ± 10.1% (mean ± standard deviation) compared to the phagocytosis in the controls, which is significantly less (P < 0.02; n = 4) than the 49.0% ± 4.4% reduction in phagocytosis recorded with early-stage GEs. PI, phagocytic index.
PPARγ-RXR agonists upregulate CD36 in human Mφs and increase their phagocytic capacity for GEs.
Human Mφs were incubated for 48 h with PPARγ-RXR agonists (5 μM 15d-PGJ2 and 1 μM 9-cis-RA) and processed for flow cytometry. Data from the flow cytometric analysis (Fig. 5A) revealed that CD36 levels were elevated on Mφs that were incubated with PPARγ-RXR agonists. Phagocytosis assays were carried out by using Mφs that had been incubated for 48 h with PPARγ-RXR agonists. Figure 5B shows that phagocytosis of nonopsonized erythrocytes containing stage I and IIA gametocytes was increased in treated Mφs by ∼70% compared to the controls (P < 0.01; n = 3). Blocking CD36 with 10 μg of FA6 per ml in both untreated and treated Mφs resulted in significant reductions in phagocytosis (53.9 and 52.0%, respectively) compared to the phagocytosis in the controls (P < 0.01; n = 3).
Murine CD36 mediates the uptake of GEs by murine Mφs.
Erythrocytes containing asexual stages of P. falciparum have previously been shown to adhere to murine CD36 on the surfaces of COS-7 cells (39). As an alternative experimental strategy to examine the role of CD36 in mediating the uptake of GEs by Mφs, we examined the phagocytosis of GEs by wild-type and CD36-null murine Mφs. Wild-type or CD36-null thioglycolate-induced murine Mφs were incubated with anti-murine CD36 prior to phagocytosis assays performed with untreated or trypsin-treated cultures. Typically, ∼20% of wild-type murine Mφs internalized at least one nonopsonized GE. Figure 6 shows that phagocytosis of GEs by CD36-null murine Mφs was reduced by ∼50% compared to phagocytosis of GEs by wild-type murine Mφs. Furthermore, phagocytosis of GEs by wild-type murine Mφs treated with anti-murine CD36 was reduced by ∼50%, which is equivalent to the reduction observed in CD36-null Mφs. CD36 blockade of CD36-null murine Mφs did not significantly reduce phagocytosis compared to phagocytosis by control CD36-null murine Mφs.
DISCUSSION
In this study, we obtained several lines of evidence demonstrating that the phagocytosis by human monocytes and culture-derived Mφs of nonopsonized erythrocytes containing P. falciparum stage I and IIA gametocytes is mediated in large part by the scavenger receptor CD36. Fluorescent microscopy revealed that GEs are internalized by monocytes and Mφs with relatively high frequency (∼20 to 25% of phagocytes internalized at least one GE). Blocking CD36 with monoclonal antibodies specific to this receptor reduced phagocytosis of GEs by almost ∼50%, whereas cleaving trypsin-sensitive ligands (including PfEMP-1), which are known to mediate binding to CD36, reduced uptake by ∼80%. Thioglycolate-induced murine Mφs were also able to internalize human erythrocytes containing P. falciparum stage I and IIA gametocytes, and this uptake was reduced by ∼50% in CD36-null murine Mφs. Finally, we show that treating Mφs with PPARγ-RXR agonists increased by ∼70% the phagocytosis of GEs via the pharmacological upregulation of CD36. This clearance mechanism and upregulation of phagocytosis are similar to those observed with asexual parasites (28, 40); however, internalization of erythrocytes infected with stage I and IIA gametocytes represents a previously unrecognized clearance pathway that may have transmission implications.
Little is known about the mechanisms by which gametocytes of Plasmodium species are cleared or destroyed by the human immune system. Most in vitro studies of the phagocytosis (opsonic or nonopsonic) or destruction (mediated by free radicals or cytokines) of P. falciparum-infected erythrocytes have focused primarily on asexual stages of the parasite (10, 15, 20, 50, 52, 56). Sinden and Smalley (43) described the in vitro phagocytosis by various leukocytes of extracellular mature gametocytes (stage V) that had been isolated from the guts of freshly fed mosquitoes. Lensen and colleagues (26, 27) attempted to correlate opsonic phagocytosis of gametes in the mosquito midgut by leukocytes with transmission-blocking activity. However, in neither of these studies did the researchers examine phagocytosis of early-stage GEs.
Healer and colleagues (23) investigated phagocytosis of various stages of P. falciparum, including schizonts, gametocytes, and gametes, by human leukocytes. Using in vitro phagocytosis assays, they found that nonopsonized or opsonized erythrocytes containing mature (stage V) gametocytes were not internalized to any significant degree by neutrophils or by monocytes and Mφs. Based on this result, they surmised that mature gametocytes have few, if any, parasite-specific antigens expressed on the erythrocyte surface and thus that an antibody-mediated immune response could not be mounted against these stages. Based on our study, however, a lack of parasite-specific ligands might instead result in the inability of a monocyte or Mφ to recognize and bind a nonopsonized erythrocyte infected with a mature gametocyte and subsequently to internalize it. In addition, since we have shown that immature gametocytes are readily ingested by monocytes and Mφs, the conclusion of Healer et al. that phagocytosis of gametocytes does not play a role in blocking transmission of P. falciparum may now be modified.
We observed that phagocytosis of nonopsonized erythrocytes containing stage I and IIA gametocytes was significantly reduced by removing parasite ligands (including PfEMP-1) and by antibody blockade of CD36 expressed on monocytes and Mφs. These results suggest that internalization of these early sexual stages was largely dependent upon an initial binding interaction between PfEMP-1 and CD36 and subsequent phagocytosis by a CD36-mediated pathway. Although uncharacterized at the time, such a nonopsonic mechanism was observed by Ferrante and colleagues (21), who reported that phagocytosis of asexual stages of P. falciparum by monocytes and Mφs occurred with equal avidity in the absence and in the presence of opsonins (human immune sera), whereas phagocytosis of parasites by neutrophils was almost completely reliant on the presence of opsonins in human immune sera.
The potential for a beneficial role for CD36 in malaria, as suggested by this study and others (2, 40), contrasts with recent strategies to target the interaction between PEs and CD36 (6, 17). Based on our data, blocking the GE-CD36 interaction might be expected to reduce the number of gametocytes internalized by phagocytic cells, thus increasing the number of sexual stages present in the bloodstream and therefore transmission. These considerations, along with the observation that little CD36 is present on cerebral microvasculature and the observation that only a minority of infected nonimmune patients develop severe malaria (even though most wild isolates of P. falciparum adhere to CD36), suggest that the PE-CD36 interaction may represent a complex coevolutionary adaptation of the parasite and host (40).
The reduction in phagocytosis of GEs by monocytes and Mφs by anti-CD36 monoclonal antibody blockade (∼50%) was not as complete as that observed for asexual parasites (∼70% inhibition by anti-CD36 antibodies). In addition, the increase in phagocytosis of GEs by Mφs previously incubated with PPARγ-RXR agonists (∼70%) was not as great as that observed for asexual parasites (∼125%) (40). From these results, it appears that CD36 is the primary receptor mediating binding and phagocytosis of GEs by monocytes and Mφs. However, one or more as-yet-unknown receptors are responsible for the other 50% of binding and internalization. Whether PfEMP-1 or other molecules, the ligands that mediate adherence to these unidentified receptors are largely trypsin sensitive, as indicated by the 80% inhibition of phagocytosis caused by protease pretreatment of cultures containing stage I and IIA gametocytes. At stage IIB, unidentified receptors were responsible for the vast majority of binding and phagocytosis of gametocytes, indicating that ligand-receptor complexes change greatly throughout the course of gametocytogenesis. Hayward and colleagues (22) observed that PfEMP-1 was expressed during stages I and IIA and bound to CD36 in a manner similar to that of asexual parasites. However, they found that PfEMP-1 expression abruptly ceased at stage IIB. Rogers and colleagues (37) showed that later gametocytes (stage III and IV gametocytes) cytoadhered to human bone marrow cell lines and that a PfEMP-1-CD36 interaction was not involved in this binding. Concomitant with this change in ligands expressed on GEs is a switch in sequestration sites from typical sites for asexual parasites to various cell types of the spleen and bone marrow (44, 45).
The CD36 promoter contains a binding site for the PPARγ-RXR transcriptional activator complex. When the PPARγ agonist 15d-PGJ2 and the RXR agonist 9-cis-RA bind to this receptor complex, CD36 gene expression in Mφs is upregulated and CD36 surface levels increase (48). The increase in CD36 results in significantly increased phagocytic capacity of human monocytes and monocyte-derived Mφs for asexual parasites (40) and gametocytes (this study). In the case of asexual parasites, PPARγ-RXR activation also caused a decrease in parasite-induced production of TNF-α (40).
Vitamin A supplementation has been shown to potentiate host resistance to malaria. Recent data indicate that the beneficial effects may be mediated, at least in part, by the action of 9-cis RA, a metabolite of vitamin A, on PPARγ-RXR, resulting in increased clearance of asexual PEs and decreased proinflammatory responses to infection (41). The findings of the present study suggest that vitamin A supplementation programs might be expected to have an impact on clearance of early sexual stages and malaria transmission; this question can now be addressed in vivo.
There are potential in vivo consequences of our in vitro data. Specifically, could the pharmacological modulation of the phagocytic capacity of monocytes and Mφs represent a novel means to facilitate clearance of gametocytes and thus decrease transmission of P. falciparum? Theoretically, only one male gametocyte and one female gametocyte in a single mosquito blood meal are required to form an oocyst. The majority of human infections with P. falciparum are characterized by a gametocytemia, if it exists, that is at least 1 order of magnitude less than the level of asexual parasitemia (47). In addition, gametocytemia is generally correlated with the number of oocysts in mosquitoes that feed on infected humans (13). An argument in favor of pharmacological upregulation of phagocytosis is that since the numbers of gametocytes ingested by a mosquito are already very low, increasing the phagocytosis of early stages of gametocytes by 70% (as observed in this study) may be sufficient to block transmission. Gametes from male gametocytes must be highly efficient at finding females in a large blood meal in order to form ookinetes (18, 54). Nevertheless, a mathematical model that incorporates parameters such as gametocytemia in the blood and the size of a blood meal taken by a mosquito suggests that a threshold number of male and female gametocytes (i.e., more than 20 gametocytes) is required to ensure production of at least one viable oocyst (55). Reducing the gametocytemia to a level below this threshold may prevent the formation or at least greatly reduce the number of oocysts formed in a mosquito.
Recent research on the sexual stages of P. falciparum has concentrated on the development of gametocyte-specific drug therapies and the characterization of gametocyte and gamete antigens for the purpose of developing a transmission-blocking vaccine (24). However, it is evident from this study that innate immunity may also play a role in determining the number of circulating gametocytes available to be ingested by mosquitoes. The in vitro data presented here need to be confirmed by in vivo studies in order to determine the role of this clearance mechanism in decreasing transmission of P. falciparum.
Acknowledgments
This work was supported by Canadian Institutes of Health Research operating grant MT-13721 (K.C.K.), by a Canada Research Chair (K.C.K.), and by a Career Scientist Award from the Ontario Ministry of Health (K.C.K.). T.G.S. is the recipient of a postdoctoral fellowship from the Canadian Blood Services, and L.S. is the recipient of a studentship from the Canadian Institutes of Health Research.
We thank David Baker, Geoff Targett, Lisa Ranford-Cartwright, and David Walliker for providing and transporting antibody 93A3 and Annie-Claude Labbé, Thomas Whitehead, and Yvonne Yau for drawing blood from some of us for monocyte preparation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aikawa, M., M. Iseki, J. W. Barnwell, D. Taylor, M. M. Oo, and R. J. Howard. 1990. The pathology of human cerebral malaria. Am. J. Trop. Med. Hyg. 43:30-37. [DOI] [PubMed] [Google Scholar]
- 2.Aitman, T. J., L. D. Cooper, P. J. Norsworthy, F. N. Wahid, J. K. Gray, B. R. Curtis, P. M. McKeigue, D. Kwiatkowski, B. M. Greenwood, R. W. Snow, A. V. Hill, and J. Scott. 2000. Malaria susceptibility and CD36 mutation. Nature 405:1015-1016. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1999. Malaria, 1982-1997. W. H. O. Wkly. Epidemiol. Rec. 74:265-270. [PubMed] [Google Scholar]
- 4.Baker, D. A., O. Daramola, M. V. McCrossan, J. Harmer, and G. A. T. Targett. 1994. Subcellular localization of Pfs16, a Plasmodium falciparum gametocyte antigen. Parasitology 108:129-137. [DOI] [PubMed] [Google Scholar]
- 5.Barnwell, J. W., and M. R. Galinski. 1998. Invasion of vertebrate cells: erythrocytes, p. 93-120. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 6.Baruch, D. I., J. A. Gormley, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intracellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch, D. I., X. C. Ma, H. B. Singh, X. Bi, B. L. Pasloske, and R. J. Howard. 1997. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood 90:3766-3775. [PubMed] [Google Scholar]
- 8.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berendt, A. R., D. Simmons, J. Tansey, C. K. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule 1 (ICAM-1) is an endothelial cytoadherence receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 10.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce, M. C., D. A. Baker, P. Alano, N. C. Rogers, P. M. Graves, G. A. Targett, and R. Carter. 1990. Sequence coding for a sexual stage specific protein of Plasmodium falciparum. Nucleic Acids Res. 18:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce, M. C., N. C. Carter, K. Nakamura, M. Aikawa, and R. Carter. 1994. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol. Biochem. Parasitol. 65:11-22. [DOI] [PubMed] [Google Scholar]
- 13.Carter, R., and P. M. Graves. 1988. Gametocytes, p. 253-305. In W. H. Wernsdorfer and I. A. McGregor (ed.), Malaria: principles and practice of malariology. Churchill Livingstone, Edinburgh, Scotland.
- 14.Carter, R., L. C. Ranford-Cartwright, and P. Alano. 1993. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies, p. 67-88. In J. E. Hyde (ed.), Methods in molecular biology, vol. 21. Protocols in molecular parasitology. Humana Press, Totawa, N.J. [DOI] [PubMed]
- 15.Celada, A., A. Cruchaud, and L. H. Perrin. 1983. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. J. Parasitol. 69:49-53. [PubMed] [Google Scholar]
- 16.Chiayaroj, S. C., P. Angkasekwinai, A. Buranakiti, S. Looareesuwan, S. J. Rogerson, and G. V. Brown. 1996. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate as a cytoadherence receptor. Am. J. Trop. Med. Hyg. 55:76-80. [DOI] [PubMed] [Google Scholar]
- 17.Cooke, B. M., C. L. Nicoll, D. I. Baruch, and R. L. Coppel. 1998. A recombinant peptide based on PfEMP-1 blocks and reverses adhesion of malaria-infected red blood cells to CD36 under flow. Mol. Microbiol. 30:83-90. [DOI] [PubMed] [Google Scholar]
- 18.Day, K. P., R. E. Hayward, and M. Dyer. 1998. The biology of Plasmodium falciparum transmission stages. Parasitology 116:S95-S109. [DOI] [PubMed] [Google Scholar]
- 19.Deitsch, K. W., and T. E. Wellems. 1996. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol. Biochem. Parasitol. 76:1-10. [DOI] [PubMed] [Google Scholar]
- 20.Febbraio, M., N. A. Abumrad, D. P. Hajjar, K. Sharma, W. Cheng, S. F. A. Pearce, and R. L. Silverstein. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274:19055-19062. [DOI] [PubMed] [Google Scholar]
- 21.Ferrante, A., L. Kumaratilake, C. M. Rzepczyk, and J.-M. Dayer. 1990. Killing of Plasmodium falciparum by cytokine-activated effector cells (neutrophils and macrophages). Immunol. Lett. 25:179-188. [DOI] [PubMed] [Google Scholar]
- 22.Hayward, R. E., B. Tiwari, K. P. Piper, D. I. Baruch, and K. P. Day. 1999. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 96:4563-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healer, J., A. Graszynski, and E. Riley. 1999. Phagocytosis does not play a major role in naturally acquired transmission-blocking immunity to Plasmodium falciparum malaria. Infect. Immun. 67:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow, D. C. 1998. Acquired immunity to sexual stages, p. 457-466. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 25.Kersten, S., B. Desvergne, and W. Wahli. 2000. Roles of PPARs in health and disease. Nature 405:421-424. [DOI] [PubMed] [Google Scholar]
- 26.Lensen, A., L. Mulder, T. Tchuinkam, L. Willemsen, W. Eling, and R. Sauerwein. 1998. Mechanisms that reduce transmission of Plasmodium falciparum malaria in semiimmune and nonimmune persons. J. Infect. Dis. 177:1358-1363. [DOI] [PubMed] [Google Scholar]
- 27.Lensen, A. H. W., M. Bolmer-Van de Vegte, G. J. Van Gemert, W. M. C. Eling, and R. W. Sauerwein. 1997. Leukocytes in a Plasmodium falciparum-infected blood meal reduce transmission of malaria to Anopheles mosquitoes. Infect. Immun. 65:3834-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGilvray, I. D., L. Serghides, A. Kapus, O. D. Rotstein, and K. C. Kain. 2000. Non-opsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitised erythrocytes: a role of CD36 in malarial clearance. Blood 96:3231-3240. [PubMed] [Google Scholar]
- 29.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 30.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 31.Newbold, C. I., G. Warn, A. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 57:389-398. [DOI] [PubMed] [Google Scholar]
- 32.Ockenhouse, C. F., and J. D. Chulay. 1988. Plasmodium falciparum sequestration: OKM5 antigen (CD36) mediates cytoadherence of parasitized erythrocytes to a myelo-monocytic cell line. J. Infect. Dis. 157:584-588. [DOI] [PubMed] [Google Scholar]
- 33.Ockenhouse, C. F., M. Ho, N. N. Tandon, G. A. Van Seventer, S. Shaw, N. J. White, G. A. Jamieson, J. D. Chulay, and H. K. Webster. 1991. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J. Infect. Dis. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 34.Ockenhouse, C. F., C. Magowan, and J. D. Chulay. 1989. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J. Clin. Investig. 84:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oquendo, P., E. Hundt, J. Lawler, and B. Seed. 1989. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 58:95-101. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, D. D., J. A. Sherwood, S. L. Spitalnik, L. J. Panton, R. J. Howard, V. M. Dixit, W. A. Frazier, L. H. Miller, and V. Grinsburg. 1985. Thrombospondin binds falciparum parasitized erythrocytes and may mediate cytoadherence. Nature 318:64-66. [DOI] [PubMed] [Google Scholar]
- 37.Rogers, N. J., B. S. Hall, J. Obiero, G. A. T. Targett, and C. J. Sutherland. 2000. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect. Immun. 68:3455-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savill, J., N. Hogg, Y. Ren, and C. Haslett. 1992. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Investig. 90:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serghides, L., I. C. Crandall, E. Hull, and K. C. Kain. 1998. The Plasmodium falciparum-CD36 interaction is modified by a single amino acid substitution in CD36. Blood 92:1814-1819. [PubMed] [Google Scholar]
- 40.Serghides, L., and K. C. Kain. 2001. PPARγ-RXR agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNFα secretion by monocytes/macrophages. J. Immunol. 166:6742-6748. [DOI] [PubMed] [Google Scholar]
- 41.Serghides, L., and K. C. Kain. 2002. Mechanism of vitamin A-induced protection in falciparum malaria. Lancet 359:1404-1406. [DOI] [PubMed] [Google Scholar]
- 42.Siano, J. P., K. K. Grady, P. Millet, and T. M. Wick. 1998. Short report. Plasmodium falciparum: cytoadherence to αvβ3 on human microvascular endothelial cells. Am. J. Trop. Med. Hyg. 59:77-79. [DOI] [PubMed] [Google Scholar]
- 43.Sinden, R. E., and M. E. Smalley. 1976. Gametocytes of Plasmodium falciparum: phagocytosis by leucocytes in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 70:344-345. [DOI] [PubMed] [Google Scholar]
- 44.Smalley, M. E. 1976. Plasmodium falciparum gametocytogenesis in vitro. Nature 264:271-272. [DOI] [PubMed] [Google Scholar]
- 45.Smalley, M. E., S. Abdalla, and J. Brown. 1980. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R. Soc. Trop. Med. Hyg. 75:103-105. [DOI] [PubMed] [Google Scholar]
- 46.Smith, T. G., P. Lourenco, R. Carter, D. Walliker, and L. C. Ranford-Cartwright. 2000. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121:127-133. [DOI] [PubMed] [Google Scholar]
- 47.Taylor, L. H., and A. F. Read. 1997. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol. Today 13:135-140. [DOI] [PubMed] [Google Scholar]
- 48.Tontonoz, P., L. Nagy, J. G. Alvarez, V. A. Thomazy, and R. M. Evans. 1998. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241-252. [DOI] [PubMed] [Google Scholar]
- 49.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-677. [DOI] [PubMed] [Google Scholar]
- 50.Trubowitz, S., and B. Mazek. 1968. Plasmodium falciparum: phagocytosis by polymorphonuclear leukocytes. Science 162:273-274. [DOI] [PubMed] [Google Scholar]
- 51.Turner, G. D., H. Morrison, and M. Jones. 1994. An immunohistochemical study of the pathology of fatal malaria. Am. J. Pathol. 145:1057-1069. [PMC free article] [PubMed] [Google Scholar]
- 52.Turrini, F., H. Ginsburg, F. Bussolino, G. P. Pescarmona, M. V. Serra, and P. Arese. 1992. Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes: involvement of immune and non-immune determinants and dependence on parasite developmental stage. Blood 80:801-808. [PubMed] [Google Scholar]
- 53.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]
- 54.West, S. A., T. G. Smith, and A. F. Read. 2000. Sex allocation and population structure in apicomplexan (protozoa) parasites. Proc. R. Soc. Lond. B 267:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West, S. A., T. G. Smith, S. R. Lee, and A. F. Read. 2002. Fertility insurance and the sex ratios of malaria and related haemospororin blood parasites. J. Parasitol. 88:258-263. [DOI] [PubMed] [Google Scholar]
- 56.Wozencraft, A. O., H. M. Dockrell, J. Taverne, G. A. T. Targett, and J. H. L. Playfair. 1984. Killing of human malaria parasites by macrophage secretory products. Infect. Immun. 43:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]