Abstract
LuxS is responsible for the production of autoinducer 2 (AI-2), which functions in Vibrio harveyi as a quorum-sensing signal that controls the cell density-dependent expression of the lux operon. In nonluminescent organisms, the physiologic role of AI-2 is not clear. We report that inactivation of luxS in Actinobacillus actinomycetemcomitans JP2 results in reduced growth of the mutant, but not the wild-type organism, under aerobic, iron-limited conditions. Stunted cultures of the luxS mutant A. actinomycetemcomitans JP2-12 grew to high cell density when subcultured under iron-replete conditions. In addition, the mutant strain grew to high cell density under iron limitation after transformation with a plasmid containing a functional copy of luxS. Results of real-time PCR showed that A. actinomycetemcomitans JP2-12 exhibited significantly reduced expression of afuA (eightfold), fecBCDE (10-fold), and ftnAB (>50-fold), which encode a periplasmic ferric transport protein, a putative ferric citrate transporter, and ferritin, respectively. The expressions of putative receptors for transferrin, hemoglobin, and hemophore binding protein were also reduced at more modest levels (two- to threefold). In contrast, expressions of sidD and frpB (encoding putative siderophore receptors) were increased 10- and 3-fold, respectively, in the luxS mutant. To better understand the mechanism of the AI-2 response, the A. actinomycetemcomitans genome was searched for homologs of the V. harveyi signal transduction proteins, LuxP, LuxQ, LuxU, and LuxO. Interestingly, ArcB was found to be most similar to LuxQ sensor/kinase. To determine whether arcB plays a role in the response of A. actinomycetemcomitans to AI-2, an arcB-deficient mutant was constructed. The isogenic arcB mutant grew poorly under anaerobic conditions but grew normally under aerobic iron-replete conditions. However, the arcB mutant failed to grow aerobically under iron limitation, and reverse transcriptase PCR showed that inactivation of arcB resulted in decreased expression of afuA and ftnAB. Thus, isogenic luxS and arcB mutants of A. actinomycetemcomitans exhibit similar phenotypes when cultured aerobically under iron limitation, and both mutants exhibit reduced expression of a common set of genes involved in the transport and storage of iron. These results suggest that LuxS and ArcB may act in concert to control the adaptation of A. actinomycetemcomitans to iron-limiting conditions and its growth under such conditions.
Cell density-dependent regulation of bacterial gene expression is known as quorum sensing (2, 14, 23). Quorum-sensing bacteria synthesize and secrete extracellular signaling molecules called autoinducers (AI), which accumulate in the environment as the bacterial population increases. When a critical threshold concentration of AI is attained, a signal transduction cascade is triggered, resulting in an alteration in gene expression that may influence the virulence or lifestyle of the organism (1, 2, 13, 14, 23, 37, 45, 49, 64, 67). Several independent quorum-sensing systems have been identified in gram-negative bacteria. One type of quorum-sensing system requires an acyl-homoserine lactone signal and has been well characterized in Pseudomonas aeruginosa (14, 23, 47), Agrobacterium tumefaciens (45), Burkholderia cepacia (37, 38), Vibrio fischeri (16, 17, 53), and other gram-negative bacteria. In these organisms, specific genes, e.g., luxRI, lasRI, and cepRI, are dedicated to the synthesis and detection of the acyl-homoserine lactone signal (23, 37, 47, 48).
A second type of quorum-sensing system has been most thoroughly characterized in Vibrio harveyi (2, 54) and responds to a bicyclic autoinducer signal (AI-2) that is produced by the LuxS protein and contains boron (11). In V. harveyi, AI-2 is bound by LuxP (11), the LuxQ sensor kinase/phosphatase. At low cell density, and hence low AI-2 concentration, LuxQ functions as a kinase and autophosphorylates (39). Phosphate is then transferred to LuxU, which in turn donates phosphate to the response regulator protein, LuxO (6, 19, 21). Phosphorylated LuxO is active and influences colony morphology and the production of siderophore by V. harveyi (39). Activated LuxO also presumably induces the expression or activity of an unidentified repressor of the luciferase structural operon, luxCDABE (39). At high cell density, LuxQ functions as a phosphatase, which draws phosphate away from LuxO in a LuxU-dependent reaction. Thus, LuxO becomes inactive, and the downstream repression of luxCDABE is removed, resulting in the production of light. In other bacteria, LuxS-dependent signaling has been shown to regulate the expression of various virulence factors, secretion systems, regulatory proteins, and polypeptides that are involved in the acquisition of hemin (10, 12, 18, 40, 61-63, 65, 66). Indeed, the expression of up to 400 genes in Escherichia coli may be influenced by AI-2 (15, 61). However, although the expression of many genes has been shown to respond to AI-2, the complex physiologic responses induced by AI-2 in these organisms remain largely uncharacterized.
Interestingly, LuxS appears to be present in all bacteria and AI-2 is capable of inducing both intra- and interspecies responses (3, 18). This has led to speculation that AI-2 may function to report the total cell density and/or the metabolic potential of a bacterial community (2, 67). However, recent studies have shown that LuxS catalyzes the cleavage of S-ribosylhomocysteine to generate homocysteine and 4,5-dihydroxy-2,3-pentanedione (DHP) (55, 70). DHP is then thought to spontaneously form 4-hydroxy-5-methyl-3(2H)-furanone (MHF), which is a precursor of AI-2. Thus, LuxS may not be dedicated solely to AI-2 synthesis since it may also play an important metabolic role in the activated methyl cycle (55, 70). This led Winzer et al. to suggest that AI-2 may not be a signal involved in cell-to-cell communication in all bacteria but rather may represent a metabolite that is secreted early in growth and consumed later (70, 71). Furthermore, the degree to which the structure of AI-2 is conserved is not known. The boron in AI-2 produced by V. harveyi has been suggested to arise from the reaction of boric acid with pro-AI-2 (11). However, boric acid is present at a significantly higher concentration in the marine environment than in freshwater (22, 71). Thus, it is possible that the structure and function of AI-2 produced by Actinobacillus actinomycetemcomitans under physiologic conditions may differ from those of V. harveyi AI-2.
A. actinomycetemcomitans is a capnophilic gram-negative bacterium that is associated with various forms of early-onset periodontal diseases (60, 73, 74), endocarditis (7), and subcutaneous abscesses (46). The mechanisms of A. actinomycetemcomitans pathogenesis are not well understood, but the organism expresses a variety of potential virulence-associated factors, e.g., a collagenase (51), lipopolysaccharide (32, 34), and GroEL-like proteins (26), that may stimulate the breakdown of the extracellular matrix and the induction of bone resorption. A. actinomycetemcomitans also produces several toxins which target various components of the immune system and may play a role in modulating the host response. An immunosuppressive protein related to the cytolethal distending toxins has been shown to induce G2 arrest of human lymphocytes (57, 58), and membranolytic leukotoxin of the RTX family of cytolytic toxins (31, 36) kills human cells of the lymphocytic and monomyelocytic lineages (33). Previous studies have shown that A. actinomycetemcomitans possesses luxS and produces an AI-2-like signal (16, 18). Furthermore, the addition of AI-2 to early-exponential-phase A. actinomycetemcomitans cells induced the expression of leukotoxin and a ferric ion ABC-type transporter (18). AI-2 from A. actinomycetemcomitans also complemented a LuxS deficiency in Porphyromonas gingivalis when added in trans to cultures of a P. gingivalis luxS knockout strain (18), suggesting that organisms living in a complex microbial biofilm like dental plaque may sense and respond to both cognate and heterologous AI-2 signals. In this report, we show that luxS is important for aerobic growth of A. actinomycetemcomitans under iron limitation and that the inactivation of luxS alters the expression of genes encoding several iron transport systems and iron storage proteins. Our results also suggest that this LuxS-dependent response may require the ArcB sensor/kinase of A. actinomycetemcomitans. Inactivation of arcB alters the expression of iron acquisition genes that are regulated by luxS and also gives rise to a phenotype that is similar to that of a LuxS null strain of A. actinomycetemcomitans. This suggests that ArcB may contribute to the signal transduction cascade that directs the response of A. actinomycetemcomitans to AI-2.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The A. actinomycetemcomitans strains used in this study are listed in Table 1. A. actinomycetemcomitans JP2 was grown in brain heart infusion (BHI; Difco, Detroit, Mich.) supplemented with 40 mg of NaHCO3 per liter. Cultures were maintained at 37°C in an atmosphere of 5% CO2. Recombinant A. actinomycetemcomitans strains were cultured in the same medium supplemented with ampicillin (50 μg per ml), kanamycin (25 μg per ml), or streptomycin (50 μg per ml). For some experiments, A. actinomycetemcomitans was grown in BHI under anaerobic conditions at 37°C in a ThermaForma model 1025 anaerobic workstation. Growth of A. actinomycetemcomitans under iron limitation was carried out in BHI medium supplemented with 0.1, 0.25, or 0.5 mM ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDHA; Sigma Chemical Co., St. Louis, Mo.). EDDHA was incubated overnight in the medium at 37°C prior to inoculation with bacteria. For growth studies, all cultures were inoculated at the identical cell density (2 × 108 CFU/ml) and were incubated at 37°C for 7 h. The optical density at 600 nm of each culture was measured using a Spectronic Unicam GeneSys8 spectrophotometer. Cell density, expressed in CFU per milliliter, was calculated using standard curves of the optical density at 600 nm versus CFU (per milliliter) generated for each of the A. actinomycetemcomitans strains. Standard curves for each strain were generated by removing aliquots of the A. actinomycetemcomitans cultures at various times during growth and plating in triplicate on BHI agar plates. In some experiments, BHI medium containing EDDHA was supplemented with 100 μM FeSO4 or 100 μM FeCl3 immediately prior to inoculation with A. actinomycetemcomitans. All growth experiments were repeated at least three times with consistent results. E. coli strains were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl) with aeration at 37°C. For recombinant strains carrying plasmids, antibiotic selection was carried out by supplementing LB medium with the appropriate antibiotic at the concentration indicated above.
TABLE 1.
Bacterial strains
| Strain | Reference | Description |
|---|---|---|
| A. actinomycetemcomitans JP2 | 9 | Highly leukotoxic strain |
| A. actinomycetemcomitans JP2-12 | 18 | JP2 luxS::kana |
| A. actinomycetemcomitans JP2-750 | This study | JP2-12 complemented with pJRD215luxS |
| A. actinomycetemcomitans JP2arcB1 | This study | JP2 arcB::kana |
| A. actinomycetemcomitans JP2arcB1YGS | This study | JP2arcB1 complemented with pYGSarcB |
| E. coli YGS | This study | DH5α carrying pYGS |
| E. coli AIS | 18 | DH5α carrying pGEM-TluxS |
Complementation of A. actinomycetemcomitans JP2-12.
Fong et al. previously reported the construction of an isogenic luxS-deficient strain of A. actinomycetemcomitans JP2 and showed that the knockout strain induced significantly lower levels of bioluminescence from a V. harveyi reporter than did the parent organism (18). Complementation of A. actinomycetemcomitans JP2-12 was carried out by the introduction of a functional, plasmid-borne copy of luxS. The luxS gene was obtained from E. coli AIS (18) plasmid pGEMT750 (16). A 750-bp insert containing luxS and its promoter was isolated from pGEMT750 after digestion with EcoRI (18). The resulting fragment was ligated into the unique EcoRI site of pJRD215 and introduced into competent E. coli DH5α to generate E. coli pJRD750. The broad-host-range plasmid pJRD215 is stably maintained in A. actinomycetemcomitans JP2. Therefore, pJRD750 was subsequently introduced into A. actinomycetemcomitans JP2-12 by electroporation, and recombinant clones were selected on BHI agar supplemented with kanamycin (25 μg/ml) and streptomycin (50 μg/ml). One clone, designated A. actinomycetemcomitans JP2-750, was chosen for further study. To confirm the presence of the intact copy of luxS, total DNA was isolated from A. actinomycetemcomitans JP2-750 and analyzed by PCR using the primers luxS5 and luxS3 (Table 2). Two amplification products, a 1,950-bp product arising from the inactivated genomic copy of luxS and a 750-bp product arising from the plasmid-borne intact copy of luxS, were obtained. Total DNA from A. actinomycetemcomitans JP2-12 produced only the 1,950-bp product.
TABLE 2.
Primers for PCRs
| Primer | Sequence | Target gene(s) | Product size (kbp) |
|---|---|---|---|
| luxS5 | 5′-TAAAGCCTGCGATTTTCCTG-3′ | luxS, luxS::kana | 0.75 |
| 1.95 | |||
| luxS3 | 5′-CTTATTGTTTTAATAAGCTTTCGTC-3′ | luxS, luxS::kana | 0.75 |
| 1.95 | |||
| arc5 | 5′-CGACGCTATGTGGATTGGG-3′ | arcB | 1.8 |
| arc3 | 5′-TTTCGCTACATCGGTTTGCC-3′ | arcB | 1.8 |
| arkn5 | 5′-AGTGCGGTCGAAATTCATTG-3′ | arcB::kana | 0.9 |
| arkn3 | 5′-CACTTTCTGGCTGGATGATGG-3′ | arcB::kana | 0.9 |
| arcp5 | 5′-GTTACACAATGATTTCATCGC-3′ | arcB | 2.3 |
| arcp3 | 5′-TTGCCGTTGCCATCGACTCC-3′ | arcB | 2.3 |
| afuA5 | 5′-CGCCGAATTCCAGGAATTAGGC-3′ | afuA | 0.5 |
| afuA3 | 5′-GTGATGTCGGCGAAAGCGGC-3′ | afuA | 0.5 |
| ftn5 | 5′-AAGGTTATGAAGGTGCTGCG-3′ | ftnAB | 0.2 |
| ftn3 | 5′-CGGCAAAGGTACATTCCACT-3′ | ftnAB | 0.2 |
| sid5 | 5′-GTTCAGTCTGATTAGTTTGGC-3′ | sidD | 0.7 |
| sid3 | 5-CAACAAAGGAAGGATGGGAGCG-3′ | sidD | 0.7 |
| fecB5 | 5′-CGGTTACCGATGCAAAAGGCG-3′ | fecB | 0.5 |
| fecB3 | 5′-GCTTTTACCGCCGGAATCGCC-3′ | fecB | 0.5 |
| tbp5 | 5′-TCCGTCCGGTAATGTTGATT-3′ | tbp | 0.5 |
| tbp3 | 5′-CCTTGGGAACCACCAATATG-3′ | tbp | 0.5 |
| hbp5 | 5′-AGGTGGTTCGGTCAGTTTTG-3′ | hbp | 0.5 |
| hbp3 | 5′-AAGCGGTGGTTATCTGTTGG-3′ | hbp | 0.5 |
| hasR5 | 5′-CTACGCCAAAAAGAAGCAGG-3′ | hasR | 0.5 |
| hasR3 | 5′-CGCTTTGCGTTTCTGATGTA-3′ | hasR | 0.5 |
| frp5 | 5′-CTGGGTCAGCGTGGTTTATT-3′ | frpB | 0.5 |
| frp3 | 5′-GCCCACATCACGTTTCTTTT-3′ | frpB | 0.5 |
Inactivation of A. actinomycetemcomitans arcB.
The A. actinomycetemcomitans arcB sequence was determined from the genomic sequence of A. actinomycetemcomitans HK1651 (B. A. Roe, F. Z. Najar, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer, Actinobacillus Genome Sequencing Project, University of Oklahoma [http://www.genome.ou.edu/act.html]). A 1.8-kbp portion of arcB was amplified from A. actinomycetemcomitans JP2 genomic DNA by using primers arc5 and arc3 (Table 2) and was subsequently ligated into pGEM-T (Promega Corp., Madison, Wis.) and transformed into E. coli DH5α. The resulting plasmid, pGEMTarcB, was cleaved at the unique MfeI site within the arcB sequence and ligated with the kanamycin resistance marker obtained by EcoRI digestion of pUC4K (Amersham Pharmacia, Piscataway, N.J.). The MfeI site resides between the sequence encoding the transmitter domain of arcB and downstream receiver and phosphotransfer domains of the sensor. Thus, insertion of the resistance marker uncoupled the transmitter from the acceptor and phosphotransfer functions of ArcB. The ligation mixture was transformed into E. coli DH5α, and the desired recombinant clones were selected on LB agar containing 25 μg of kanamycin per ml and 50 μg of ampicillin per ml. The plasmid was isolated from a single recombinant organism and confirmed by restriction digestion using EcoRI. Samples of the plasmid (0.1 to 1.0 μg) were subsequently introduced into A. actinomycetemcomitans JP2 by electroporation, and recombinant A. actinomycetemcomitans clones were selected on BHI agar containing 25 μg of kanamycin per ml. Integration of the plasmid into the arcB gene of A. actinomycetemcomitans JP2 was confirmed by PCR using a forward primer (arkn5 [Table 2]) that was derived from genomic DNA sequences that reside upstream from the arc5 primer used to generate the 1.8-kbp product described above. The reverse primer used for these amplification reactions was arkn3 (Table 2), which anneals in the kanamycin resistance marker. Thus, the arkn5 and arkn3 primers generate a product only if pGEMTarcB integrates into the A. actinomycetemcomitans genome. As expected, JP2 genomic DNA failed to generate a product with arkn5 and arkn3, since JP2 genomic DNA lacks the kanamycin resistance marker. Similarly, PCRs using pGEMTarcB as the template failed to produce a product, since the plasmid construct lacks arcB sequences upstream from the arc5 primer site and cannot anneal to arkn5. However, two recombinant clones that produced the expected amplification product were identified. One of these clones, designated A. actinomycetemcomitans JP2arcB1, was chosen for further analysis.
Construction of pYGS and complementation of A. actinomycetemcomitans JP2arcB1.
To complement A. actinomycetemcomitans JP2arcB1 with a functional copy of arcB, we first constructed an E. coli and A. actinomycetemcomitans shuttle vector similar to pYGK (8) but with resistance to streptomycin specified. This was accomplished by partially digesting plasmid pJRD215 (Spr) with Sau3A, cloning the resulting fragments into BamHI-cleaved pBluescript SK, and screening for colonies exhibiting resistance to streptomycin. One resistant clone that contained a 1.5-kbp insert was chosen for further analysis. This plasmid was subsequently cleaved at the HindIII and SpeI sites that reside in the pBluescript-cloning region and flank the 1.5-kbp insert. The resulting insert fragment was blunted with T4 DNA polymerase by following established procedures (52) and ligated into Actinobacillus pleuropneumoniae plasmid pYG53 (35). The ligation mixture was transformed into E. coli DH5α, and the plasmid that was isolated from streptomycin-resistant clones was confirmed by restriction analysis and designated pYGS.
To complement A. actinomycetemcomitans JP2arcB1 with a functional copy of arcB, a 2.3-kbp fragment containing the complete arcB gene and its promoter was amplified from A. actinomycetemcomitans JP2 genomic DNA by using primers arcp5 and arcp3 (Table 2). The amplification product was ligated into pGEM-T and transformed into E. coli DH5α. The plasmid that was isolated from ampicillin-resistant recombinant E. coli clones was confirmed by restriction digestion with EcoRI. The 2.3-kbp insert was then excised and purified from an agarose gel (52) and ligated with pYGS that had been cleaved with EcoRI. The resulting plasmid, pYGSarcB, was purified from streptomycin-resistant recombinants and introduced into A. actinomycetemcomitans JP2arcB1 by electroporation. Clones exhibiting resistance to streptomycin and kanamycin were chosen for further study. The presence of pYGSarcB was confirmed by restriction digestion, and ArcB function was evaluated by examining the growth of the recombinant A. actinomycetemcomitans clones under aerobic and anaerobic conditions.
RNA isolation, RT-PCR, and real-time PCR.
A. actinomycetemcomitans total RNA was isolated from cells obtained from a 30-ml culture that was grown aerobically in BHI medium using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, Calif.) according to the manufacturer’s instructions. The RNA preparation was then digested with RQ RNase-free DNase I (Promega Corp.) to remove contaminating genomic DNA. Samples were checked for residual genomic DNA by standard PCR or by real-time PCR using the afuA5 and afuA3 primers (Table 2). RNA samples were deemed to be free of genomic DNA if no amplification product was detected by agarose gel electrophoresis (or by real-time PCR) after at least 30 cycles of amplification. Reverse transcriptase PCR (RT-PCR) reactions were performed with 100 ng of total RNA and Ready-To-Go RT-PCR beads (Amersham Pharmacia) according to the manufacturer's instructions. PCRs were performed under the following conditions: denaturation at 94°C for 50 s, annealing at 55°C for 50 s, and elongation at 72°C for 90 s for 30 cycles. For real-time RT-PCR, first-strand cDNA was prepared by using Ready-To-Go You-Prime First-Strand beads (Amersham Pharmacia) as described in the manufacturer's protocol and the appropriate gene-specific antisense primer (Table 2) and 2 μg of total RNA. The resulting cDNA was amplified by using the Smart Cycler system (Cepheid, Sunnyvale, Calif.) in a final reaction volume of 25 μl that contained 8 μl of first-strand cDNA mix, 0.3 μM concentrations of each primer, 0.5× SYBR-Green dye (Roche Applied Science, Indianapolis, Ind.), and 2.5 U of Taq DNA polymerase (Roche Applied Science). The amplification conditions for real-time PCR were as follows: denaturation at 95°C for 30 s, annealing at 62°C for 30 s, and elongation at 72°C for 30 s for 45 cycles. The gene-specific primers used in RT-PCR and in the real-time PCRs are listed in Table 2. The threshold cycle for each real-time PCR was determined from a second derivative plot of total fluorescence as a function of cycle number by using the software package supplied with the Smart Cycler system. Real-time PCRs were carried out at least twice with consistent results. After some real-time PCRs, the end-point amplification products were visualized by electrophoresis in 1% agarose gels.
RESULTS
luxS is required for A. actinomycetemcomitans growth under iron limitation.
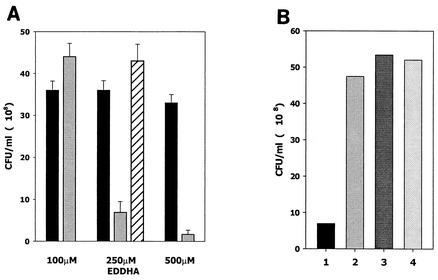
A previous study showed that inactivation of luxS in P. gingivalis altered the expression of several genes that may be involved in the acquisition of hemin (12). In addition, iron availability has been shown to influence the cell density-dependent expression of bioluminescence (30), and Lilley and Bassler have shown that AI-2 influences the production of siderophore in V. harveyi (39), suggesting that LuxS may regulate aspects of iron acquisition. To determine whether luxS plays a role in the regulation of iron acquisition by A. actinomycetemcomitans, we examined the growth of A. actinomycetemcomitans JP2 and an isogenic LuxS-deficient mutant, A. actinomycetemcomitans JP2-12, under conditions of iron limitation. To accomplish this, cultures were inoculated into medium that was supplemented with increasing concentrations of the ferric ion chelator EDDHA. As shown in Fig. 1A, A. actinomycetemcomitans JP2 adapted to iron limitation and its growth was unaffected by the presence of up to 500 μM EDDHA. In medium supplemented with up to 100 μM EDDHA, the LuxS-deficient strain JP2-12 also grew normally (Fig. 1A). However, in contrast to strain JP2, A. actinomycetemcomitans JP2-12 cultured under these conditions failed to grow to high cell density when subcultured for a second time in medium containing 100 μM EDDHA (data not shown). This suggests that the initial growth of A. actinomycetemcomitans JP2-12 in BHI and 100 μM EDDHA occurred at the expense of the intracellular iron stores. At higher concentrations of EDDHA, A. actinomycetemcomitans JP2-12 grew poorly relative to JP2 (Fig. 1A). The inability of A. actinomycetemcomitans JP2-12 to thrive under iron limitation resulted directly from the inactivation of luxS, since complementation of this strain with a functional plasmid-borne copy of luxS restored normal growth in BHI and 250 μM EDDHA (Fig. 1A). Furthermore, stunted A. actinomycetemcomitans JP2-12 cultures, e.g., cells grown in medium supplemented with 250 μM EDDHA, grew to high cell density when inoculated into fresh BHI medium (Fig. 1B, compare columns 1 and 2) or when cells were subcultured in medium supplemented with either 250 μM EDDHA and 100 μM FeSO4 (Fig. 1B, column 3) or 250 μM EDDHA and 100 μM FeCl3 (Fig. 1B, column 4). Thus, the luxS-dependent deficiency in the growth of A. actinomycetemcomitans JP2-12 under iron limitation was reversed by exogenous iron. These results suggest that luxS may play an important role in the adaptation and response of A. actinomycetemcomitans to iron limitation.
FIG. 1.
Inactivation of luxS results in a growth deficiency under conditions of iron limitation. (A) Cultures of A. actinomycetemcomitans JP2 (wild type; black columns), JP2-12 (LuxS deficient; gray columns), and JP2-750 (JP2-12 with plasmid-borne luxS; cross-hatched column) were inoculated at the identical cell densities and incubated at 37°C for 7 h (late exponential phase) in BHI medium that was supplemented with 100, 250, or 500 μM EDDHA. Cell density (CFU/milliliter) for each culture was determined as described in Materials and Methods. (B) Rescue of stunted A. actinomycetemcomitans JP2-12 cultures. A stunted culture of A. actinomycetemcomitans JP2-12 in BHI and 250 μM EDDHA (lane 1) grew to high cell density when subcultured for 7 h at 37°C in fresh BHI medium (lane 2) or in BHI medium containing 250 μM EDDHA and 100 μM FeSO4 (lane 3) or 250 μM EDDHA and 100 μM FeCl3 (lane 4).
Inactivation of luxS alters the expression of iron acquisition genes.
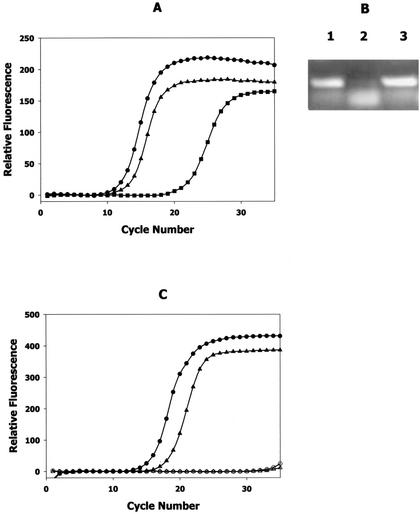
A search of the A. actinomycetemcomitans genome sequence (Actinobacillus Genome Sequencing Project, University of Oklahoma) identified numerous genes encoding proteins that exhibit similarity to known iron acquisition and storage polypeptides, including transferrin and hemoglobin receptors, siderophore receptors, multiple ferric ion transporters, and ferritin. Thus, A. actinomycetemcomitans appears to express several independent pathways for acquisition and transport of iron. To determine the molecular basis for the luxS-dependent growth deficiency described above, the expressions of genes encoding iron acquisition proteins were compared by using real-time PCR with RNA isolated from A. actinomycetemcomitans JP2 and JP2-12 cultures grown aerobically under iron-replete conditions. The results are shown in Fig. 2 and summarized in Table 3. As shown in Fig. 2A, the inactivation of luxS results in a dramatic reduction of the expression of the A. actinomycetemcomitans ferritin genes, ftnAB. The threshold cycle in the amplification of ftnAB from JP2 total RNA was determined to be cycle 12. In contrast, the threshold cycle when an equivalent amount of total RNA from A. actinomycetemcomitans JP2-12 was used was cycle 21, suggesting that the copy number of ftnAB transcript in the luxS mutant was significantly lower than that of the transcript in the wild-type strain. Consistent with this, visualization of the endpoint PCR products by gel electrophoresis after 30 cycles of amplification showed that very low levels of the primary 230-bp ftnAB product were generated from JP2-12 RNA (Fig. 2B, compare lanes 1 and 2). Melting-point analysis of the JP2-12 reaction products suggested that the smaller band obtained represented primer dimers, which likely arose from nonspecific association of the primers in the absence of the specific ftnAB RNA template. Furthermore, complementation of A. actinomycetemcomitans JP2-12 with a functional copy of luxS restored ftnAB expression nearly to the levels observed with the wild-type strain. The threshold cycle for real-time amplification reactions using RNA from A. actinomycetemcomitans JP2-750 was cycle 13 (Fig. 2A), and the amount of ftnAB product obtained at the end point of the reaction was similar to that obtained with strain JP2 (Fig. 2B, compare lanes 1 and 3). Thus, the inactivation of luxS appears to significantly reduce the expression of ftnAB in A. actinomycetemcomitans.
FIG. 2.
Inactivation of luxS results in reduced expression of ftnAB and afuA. (A) Results of real-time PCRs of DNA-free total RNA isolated from aerobic, iron-replete mid-exponential cultures of A. actinomycetemcomitans strains JP2 (•), JP2-12 (▪), and JP2-750 (▴) by using primers specific for ftnAB, encoding ferritin. (B) The endpoint products obtained from each of the real-time PCRs described above (after 30 cycles) were electrophoresed in 1.0% agarose to visualize the 230-bp DNA fragment that was generated from ftnAB. Lane 1, strain JP2; lane 2, strain JP2-12; lane 3, strain JP2-750. (C) Results of real-time PCRs of DNA-free total RNA isolated from aerobic, iron-replete mid-exponential cultures of A. actinomycetemcomitans strains JP2 (•) and JP2-12 (▴) by using primers specific for afuA, encoding a periplasmic ferric transport polypeptide. The results of control reactions that did not contain reverse transcriptase (○) or the RNA template (▵) are also shown.
TABLE 3.
Expression of iron acquisition genes in a LuxS-deficient backgrounda
| Gene | Product(s) | Expression (fold) in JP2-12b |
|---|---|---|
| ftnAB | Ferritin, iron storage protein | >−50 |
| afuA | Periplasmic ferric transport protein | −8 |
| fecB | Putative ferric citrate transport protein | −10 |
| tbp | Putative transferrin binding protein | −3 |
| hbp | Putative hemoglobin binding protein | −2 |
| hasR | Putative hemophore binding protein | −2 |
| sidD | Putative enterobactin receptor | +10 |
| frpB | Putative ferrichrome transporter | +3 |
Expression was determined by real-time PCR using primers specific for each of the indicated genes.
The levels of expression are indicated relative to those in strain JP2, which expresses a functional LuxS polypeptide (18).
Previous studies have shown that the expression of afuA, encoding a periplasmic ferric transport protein (27, 69), increased when early-exponential-phase A. actinomycetemcomitans JP2 cells were exposed to conditioned medium obtained from a high-density culture of recombinant E. coli expressing A. actinomycetemcomitans luxS (18). This suggests that the expression of afuA may be influenced by luxS and AI-2. As shown in Fig. 2C, real-time PCR analysis of total RNA from A. actinomycetemcomitans JP2 and JP2-12 showed that inactivation of luxS resulted in reduced expression of afuA. The amplification of afuA from JP2-12 RNA was first detected at cycle 17, whereas the threshold cycle in the corresponding reaction using RNA from strain JP2 was cycle 14. Thus, the copy number of the afuA transcript in the luxS mutant was decreased by approximately eightfold relative to that of the transcript in wild-type A. actinomycetemcomitans. Consistent with our results for ftnAB, complementation of A. actinomycetemcomitans JP2-12 with a functional luxS restored afuA expression (data not shown). The results of similar real-time PCRs using primers specific for other iron acquisition genes of A. actinomycetemcomitans are summarized in Table 3. The inactivation of luxS resulted in a 10-fold reduction in the expression of fecB, encoding a putative ferric citrate transport system (along with homologs of fecCDE), and more modest decreases in the expression of genes encoding putative receptors for transferrin (tbp), hemoglobin (hbp), and hemin-binding protein (hasR). In contrast, inactivation of luxS appeared to increase the expression of A. actinomycetemcomitans genes (sidD and frpB) encoding proteins that are related to known siderophore binding and transport polypeptides.
Regulation of cell growth and the expression of ftnAB and afuA by the ArcB sensor.
The common role of luxS in influencing the acquisition of iron by A. actinomycetemcomitans, P. gingivalis, and V. harveyi suggests that the mechanism for detection of and response to AI-2 may be conserved in these organisms. The AI-2 signal transduction pathway is well characterized in V. harveyi and requires the LuxP, LuxQ, LuxU, and LuxO polypeptides (19, 20, 39). A search of the A. actinomycetemcomitans genome sequence identified two polypeptides with similarity to LuxQ and three potential homologs of LuxO. However, none of these polypeptides exhibited the high level of sequence identity that was found to exist between the LuxS proteins of V. harveyi and A. actinomycetemcomitans. The A. actinomycetemcomitans proteins exhibiting the greatest similarity to LuxQ and LuxO were the ArcB sensor/kinase and a response regulator related to NtrC, respectively. Consistent with the results of a previous study by Bassler et al. (5), the LuxP protein was found to be most similar to the A. actinomycetemcomitans periplasmic ribose binding protein RbsB. Finally, no homolog of LuxU was identified in A. actinomycetemcomitans. Together, these results suggest that the response of A. actinomycetemcomitans to AI-2 may be mediated by a pathway that is significantly different from the signal transduction cascade used by V. harveyi.
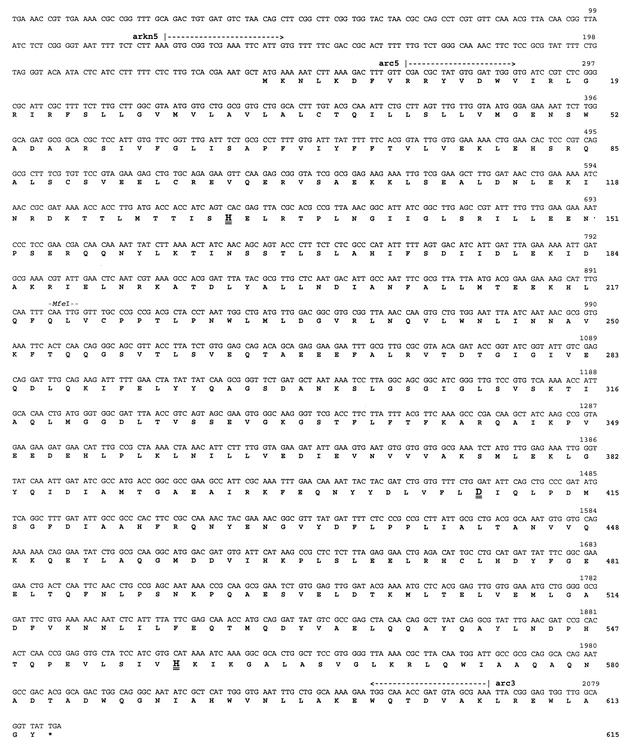
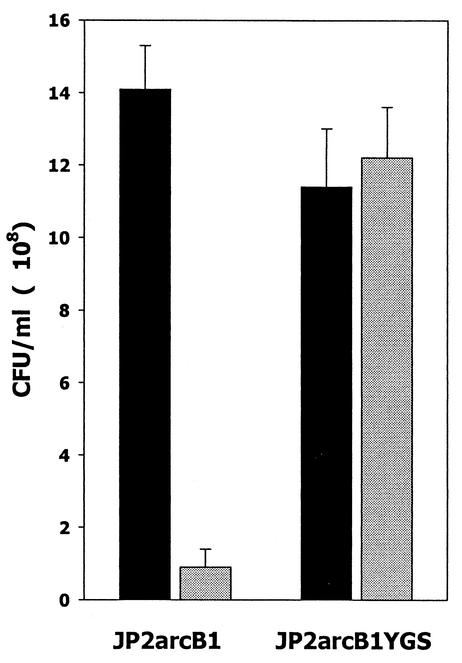
To determine whether the ArcB sensor/kinase plays a role in AI-2 signal transduction and luxS-dependent regulation of iron acquisition, an isogenic arcB mutant of A. actinomycetemcomitans JP2 was constructed. The nucleotide and deduced amino acid sequences of the arcB homolog from A. actinomycetemcomitans are shown in Fig. 3. The deduced peptide sequence of the putative A. actinomycetemcomitans ArcB exhibited 44% identity to the E. coli ArcB protein (data not shown), and residues involved in phosphotransfer reactions of E. coli ArcB were conserved in the A. actinomycetemcomitans polypeptide (Fig. 3). Inactivation of arcB was carried out by insertion of a kanamycin resistance determinant at the unique MfeI site at nucleotide 897 of the A. actinomycetemcomitans arcB sequence. Two independent clones (strains JP2arcB1 and JP2arcB2) that generated the amplification product that was predicted to arise from a Campbell-type single-recombination event of pGEMTarcB::kana at the arcB locus in A. actinomycetemcomitans were obtained. We predicted that if ArcB plays a role in transducing AI-2 signal information in A. actinomycetemcomitans, then the arcB mutant (strain JP2arcB1) would be unable to respond to AI-2 and thus would exhibit a phenotype similar to that of the luxS-deficient strain JP2-12, which does not produce AI-2. We therefore examined the growth of A. actinomycetemcomitans JP2arcB1 under iron limitation and also determined the effect of arcB inactivation on the expression of afuA and ftnAB. As shown in Fig. 4, inactivation of arcB had little effect on the aerobic growth of A. actinomycetemcomitans under iron-replete conditions (Fig. 4, compare the black columns). The slight decrease in overall cell density of the recombinant strains relative to that of strain JP2 likely arose from the presence of multiple antibiotic resistance determinants used in their construction (see Materials and Methods). Under iron-limiting conditions, A. actinomycetemcomitans JP2arcB1 grew poorly and failed to attain high cell density. However, complementation of JP2arcB1 with a functional plasmid-borne copy of arcB restored aerobic growth under iron limitation to levels observed in aerobic iron-replete cultures (Fig. 4), suggesting that the growth deficiency did not arise from polar effects of the arcB mutation. Furthermore, as shown in Fig. 5A and B, RT-PCRs using afuA- or ftnAB-specific primers suggested that inactivation of arcB resulted in significantly decreased expression of both genes, consistent with their expression in A. actinomycetemcomitans JP2-12. Real-time PCRs indicated that the expressions of afuA and ftnAB were reduced by approximately 16- and 24-fold, respectively, in JP2arcB1 and that complementation of the arcB mutant restored afuA and ftnAB expression to near wild-type levels (Table 4). These results suggest that luxS and arcB regulate a common set of genes and that they may act in concert to allow A. actinomycetemcomitans to adapt and grow under conditions of iron limitation.
FIG. 3.
Nucleotide and deduced amino acid sequences of the ArcB homolog identified from the unfinished genome of A. actinomycetemcomitans HK1651 (Actinobacillus Genome Sequencing Project, University of Oklahoma) by using the V. harveyi LuxQ peptide sequence as the probe. The nucleotide and amino acid sequences are numbered on the right. The annealing sites for PCR primers arc5, arc3, and arkn5 are indicated above the nucleotide sequence. The kanamycin resistance marker used to inactivate arcB was inserted at the unique MfeI site at nucleotide 897. Amino acid residues His131, Asp410, and His557 (shown in larger font and double underlined) correspond to residues that are involved in phosphotransfer reactions in the E. coli ArcB polypeptide.
FIG. 4.
Growth of A. actinomycetemcomitans JP2arcB1 under iron limitation. Cultures of A. actinomycetemcomitans JP2arcB1 (ArcB deficient) and JP2arcB1YGS (ArcB complementation of strain JP2arcB1) were inoculated at the identical cell density and incubated at 37°C for 7 h (late exponential phase) in BHI medium (black columns) or in BHI medium supplemented with 250 μM EDDHA (gray columns). Cell density (CFU/milliliter) for each culture was determined as described in Materials and Methods.
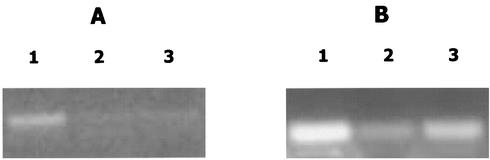
FIG. 5.
Inactivation of arcB results in reduced expression of afuA and ftnAB. Equivalent amounts of DNA-free total RNA isolated from aerobic, iron-replete cultures of A. actinomycetemcomitans JP2 (lane 1), JP2-12 (LuxS deficient; lane 2), and JP2arcB1 (ArcB deficient; lane 3) were analyzed by RT-PCR using primer specific for afuA (A) or ftnAB (B). Amplification products were visualized by ethidium bromide staining after electrophoresis in 1% agarose gels.
TABLE 4.
Expression of afuA and ftnAB in an ArcB-deficient mutant of A. actinomycetemcomitans
| Gene | Cycle threshold (Ct) for straina:
|
||
|---|---|---|---|
| JP2 | JP2arcB1 | JP2arcB1YGS | |
| afuA | 17.4 | 21.8 (≈16-fold)b | 18.4 |
| ftnAB | 12.0 | 16.5 (≈24-fold) | 12.0 |
Cycle threshold (Ct) was determined from second derivative plots of real-time PCR data obtained using the data analysis software supplied with the Smart Cycler system.
Values in parentheses indicate the approximate reduction in transcript copy number of the indicated gene in the ArcB-deficient strain JP2arcB1 relative to the numbers in strains JP2 and JP2arcB1YGS.
DISCUSSION
The widespread distribution of luxS and the observation that AI-2 is capable of inducing a response in a heterologous organism suggest that signal system 2 transcends species barriers and may function to signal the total bacterial cell density in a community and/or the metabolic potential of the environment (2, 67). However, although the expression of many genes has been shown to be influenced by AI-2 (2, 15, 39, 61), the complex physiologic responses induced by AI-2 remain largely uncharacterized in many bacteria. Our results suggest that LuxS-dependent signaling regulates the acquisition of iron by A. actinomycetemcomitans and may be important in the adaptation and subsequent growth of the organism under iron limitation. The wild-type strain, A. actinomycetemcomitans JP2, adapted well to iron-limiting conditions and grew normally in the presence of up to 0.5 mM EDDHA, suggesting that it is capable of efficiently competing with the chelator for available iron. The growth of an isogenic luxS mutant of JP2 was indistinguishable from that of the parent strain under iron-replete conditions, but it grew poorly under iron limitation. The results of real-time PCR using total RNA from the luxS mutant grown aerobically under iron-replete conditions indicated that the mutant strain expressed significantly reduced levels of several important iron acquisition genes, notably ftnAB (encoding ferritin involved in intracellular iron storage), afuA (encoding a periplasmic ferric ion binding protein (27, 69), and fecB (encoding a putative ferric citrate transport protein). Analysis of the A. actinomycetemcomitans HK1651 genome sequence (Actinobacillus Genome Sequencing Project, University of Oklahoma) indicated that afuA and fecB are both contained in operons, afuABC and fecBCDE, respectively, encoding ABC-type ferric transport systems. Thus, under laboratory conditions, the growth deficiency of the LuxS-deficient mutant under iron limitation may be explained by its reduced capacity to transport iron into the cell via the AfuABC and FecBCDE transporters and a significantly reduced capacity to store iron in intracellular ferritin complexes. Consistent with this, growth of the mutant organism under iron limitation and the expression of ftnAB and afuA were restored to levels similar to those of the parent strain JP2 when it was complemented with a functional plasmid-borne copy of luxS. However, we cannot exclude the possibility that other iron acquisition genes are down-regulated by luxS and contribute to the growth deficiency of the mutant. Indeed, our search of the A. actinomycetemcomitans genome suggested that it possesses multiple redundant systems for acquisition and transport of iron.
In vivo, A. actinomycetemcomitans must acquire iron from host proteins, e.g., transferrin and/or hemoglobin, and our results suggest that LuxS also regulates the expression of genes encoding receptors for these proteins. Interestingly, iron extracted from receptor-bound transferrin in Neisseria meningitidis is transferred to the periplasmic ferric binding protein FpbA (25), which is the N. meningitidis homolog of A. actinomycetemcomitans AfuA (25, 44). The combined decrease in the expression of tbp and afuA may thus render the luxS mutant deficient in the acquisition of iron from transferrin in vivo. Studies to examine the binding and transport of iron from transferrin by the luxS mutant and to compare the persistence and survival of A. actinomycetemcomitans JP2 and the luxS mutant in an in vivo mouse model are currently under way.
Our findings that LuxS-dependent signaling may be important in iron acquisition and storage are also consistent with those of previous studies with other bacteria that have shown that iron acquisition is closely linked to N-acyl-homoserine lactone-dependent and/or LuxS-dependent quorum-sensing systems. For example, iron availability has been shown to regulate density-dependent luminescence in V. fischeri (30) and the presence of N-acyl-homoserine lactones and siderophores has been shown to stimulate the growth of other marine bacteria (28, 29). Lewenza and Sokol have shown that the CepRI quorum-sensing system regulates siderophore biosynthesis in B. cepacia (38). In addition, inactivation of luxS in the periodontal pathogen P. gingivalis influences its growth under hemin-limiting conditions and has been shown to alter the expression of the arginine-specific protease RgpA and other outer membrane proteins that are involved in hemin acquisition (10, 12). RgpA has been suggested to increase hemin availability by degrading host hemin-sequestering polypeptides (50, 59). Finally, Lilley and Bassler have shown that AI-2 down-regulates siderophore production in V. harveyi (39). Interestingly, our analysis of spent culture medium with chromazurol S (56) suggests that A. actinomycetemcomitans produces a siderophore (D. R. Demuth, unpublished results), and real-time PCR showed that the expression of sidD, encoding a putative receptor for an enterobactin-like siderophore, is increased in the LuxS-deficient mutant of A. actinomycetemcomitans (Table 2). These results suggest that AI-2 may also function to down-regulate siderophore-mediated iron acquisition in A. actinomycetemcomitans. Studies to identify the siderophore and to isolate the genes involved in its biosynthesis to determine how luxS may regulate siderophore production in A. actinomycetemcomitans are under way.
The association of LuxS-dependent signaling with iron acquisition in A. actinomycetemcomitans, P. gingivalis, and V. harveyi suggests that the mechanism of AI-2 signal transduction may be conserved in these organisms. At present, the AI-2 signal transduction cascade has been characterized only in V. harveyi and has been shown to involve the periplasmic AI-2 receptor LuxP, the LuxQ sensor, phosphotransfer protein LuxU, and the response regulator LuxO (4, 5, 19, 20, 39). However, our search of the A. actinomycetemcomitans genome for homologs of LuxP, LuxQ, LuxU, or LuxO did not identify proteins that exhibited the high level of sequence identity that was found to occur between the V. harveyi and A. actinomycetemcomitans LuxS proteins. This suggests that the AI-2 signal transduction proteins of A. actinomycetemcomitans may have diverged in sequence from LuxP, LuxQ, LuxU, and LuxO or, alternatively, that AI-2 may not function as a cell-to-cell communication signal in A. actinomycetemcomitans. Indeed, Winzer et al. (71) have argued that the lack of highly conserved polypeptides corresponding to the signal transduction proteins of V. harveyi suggests that AI-2-dependent quorum-sensing systems do not exist in many bacteria. Instead, they propose that AI-2 represents a metabolite of the activated methyl cycle which functions as a nutrient that is secreted and subsequently consumed by the bacterial cell (71). Furthermore, Taga et al. have shown that AI-2 is actively transported into Salmonella enterica serovar Typhimurium by LsrABC and that the lsr operon is regulated by AI-2 (68). However, several other lines of evidence suggest that AI-2 does indeed function in intra- and interspecies communication. First, AI-2-dependent regulation of the lsr operon is maintained in lsr mutants of serovar Typhimurium that cannot internalize the signal (68), suggesting that these cells are still capable of sensing and responding to external AI-2. In addition, AI-2 from many diverse organisms activates quorum-sensing system 2 of V. harveyi. It has previously been shown that AI-2 from A. actinomycetemcomitans induced specific LuxS-dependent responses in a heterologous organism, P. gingivalis, and complemented a luxS mutant of P. gingivalis (18). Finally, the response of A. actinomycetemcomitans and of many other organisms to AI-2 appears to be much broader than the physiological response that would be necessary for utilization of AI-2 as a nutrient. Together, these findings suggest that AI-2 functions as a signal that is capable of inducing specific intra- and interspecies responses. Therefore, we favor the hypothesis that AI-2 signal transduction in A. actinomycetemcomitans is mediated by sensor kinase and response regulator polypeptides that have diverged in sequence from LuxQ and LuxO.
Our results show that a protein related to the ArcB sensor of A. actinomycetemcomitans exhibits the greatest similarity to LuxQ (>50% homology over 500 residues). However, although amino acid residues that are involved in the phosphotransfer reactions of E. coli ArcB are conserved in the A. actinomycetemcomitans sequence, it is not known whether A. actinomycetemcomitans ArcB is functionally equivalent to ArcB of E. coli. We also showed that growth of an isogenic arcB mutant of A. actinomycetemcomitans JP2 under aerobic, iron-limited conditions was poor relative to that of the parent strain and that the arcB mutant exhibited decreased expression of afuA and ftnAB. Thus, inactivation of arcB gave rise to a phenotype that was similar to that of the luxS mutant, and ArcB and LuxS appear to regulate a common set of genes encoding ferric transport and storage proteins. This suggests that ArcB may function as a sensor of AI-2 and may act in concert with LuxS to control the acquisition and storage of iron in A. actinomycetemcomitans during aerobic growth. Interestingly, recent studies have suggested that E. coli ArcB may have functional roles beyond controlling the response to anaerobic growth via its cognate response regulator ArcA. For example, Matsushika and Mizuno have shown that ArcB-dependent phosphotransfer reactions are complex and that ArcB may participate in more than one signaling pathway (42). ArcB has also been shown to activate the noncognate response regulators CheY (72) and OmpR (41), suggesting that ArcB-mediated cross talk may occur with other signal transduction cascades. Therefore, it is possible that E. coli ArcB mediates a broad cellular response to a variety of environmental stimuli during both aerobic and anaerobic growth. It is also interesting that ArcB is a tripartite sensor containing an integral histidine phosphotransfer domain (43, 72). Thus, AI-2 signal transduction via ArcB would not require an independent phosphotransfer polypeptide, which may explain our inability to identify a protein homologous to V. harveyi LuxU in the A. actinomycetemcomitans genome.
The level at which ArcB contributes to the putative LuxS-dependent signaling cascade in A. actinomycetemcomitans remains to be determined. It is not known whether ArcB responds to external AI-2 via a periplasmic AI-2 receptor protein (e.g., RbsB), since the periplasmic domain of ArcB is very short (approximately 10 amino acid residues). Alternatively, ArcB may interact with AI-2 after it has been internalized by the cell. Interestingly, Taga et al. have suggested that once AI-2 is transported by LsrABC, it may be modified into a molecule that functions as an internal signal (68). ArcB activity is known to respond to intracellular molecules (24), and a search of the A. actinomycetemcomitans genome indicated that the lsr operon is present in this organism (D. R. Demuth, unpublished data). Finally, it is also possible that ArcB activates only one branch of a complex signal-dependent regulatory cascade, similar to the LuxS-dependent QseB and QseC regulators of flagella and motility in E. coli (48). Clearly, defining the molecular mechanism whereby AI-2 is sensed by A. actinomycetemcomitans and identifying and characterizing the receptor and response regulator that are involved in regulating the expression of iron acquisition genes are necessary to precisely define the AI-2-dependent signal transduction cascade in A. actinomycetemcomitans.
In summary, we have shown that AI-2 is important for the adaptation of A. actinomycetemcomitans to iron limitation and its growth under such conditions and that AI-2 influences the expression of genes involved in the acquisition and storage of iron. In addition, the LuxS-dependent regulation of iron acquisition and storage genes may require the ArcB sensor.
Acknowledgments
This work was supported by Public Health Service grant DE10729 from the National Institute of Dental and Craniofacial Research.
Editor: V. J. DiRita
REFERENCES
- 1.Bainton, N. J., B. W. Bycroft, S. Chabra, P. Stead, I. Gledhill, P. J. Hill, C. E. D. Rees, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1992. A general role for the lux autoinducer in bacterial cell signaling: control of antibiotic biosynthesis in Erwinia. Gene 116:87-91. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 3.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. [DOI] [PubMed] [Google Scholar]
- 5.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 6.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol. Microbiol. 12:403-412. [DOI] [PubMed] [Google Scholar]
- 7.Block, P. J., A. C. Fox, C. Yoran, and A. J. Kaltman. 1973. Actinobacillus actinomycetemcomitans endocarditis: report of a case and review of the literature. Am. J. Med. Sci. 276:387-392. [DOI] [PubMed] [Google Scholar]
- 8.Brogan, J. M., E. T. Lally, and D. R. Demuth. 1996. Construction of pYGK, an Actinobacillus actinomycetemcomitans-Escherichia coli shuttle vector. Gene 169:141-142. [DOI] [PubMed] [Google Scholar]
- 9.Brogan, J. M., E. T. Lally, K. Poulsen, M. Kilian, and D. R. Demuth. 1994. Regulation of Actinobacillus actinomycetemcomitans expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 62:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess, N. A., D. F. Kirke, P. Williams, K. Winzer, K. R. Hardie, N. L. Meyers, J. Aduse-Opoku, M. A. Curtis, and M. Camera. 2002. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology 148:763-772. [DOI] [PubMed] [Google Scholar]
- 11.Chen, X., S. Schauder, N. Potier, A. Van Dorsselear, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 12.Chung, W., Y. Park, R. J. Lamont, R. McNab, B. Barbieri, and D. R. Demuth. 2001. A signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 183:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 14.DeKievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLisa, M. P., C.-F. Wu, J. J. Valdes, and W. E. Bentley. 2001. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J. Bacteriol. 183:5239-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap, P. V. 1999. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1:5-12. [PubMed] [Google Scholar]
- 17.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 18.Fong, K. P., W. O. Chung, R. J. Lamont, and D. R. Demuth. 2001. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect. Immun. 69:7625-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman, J. A., and B. L. Bassler. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31:665-677. [DOI] [PubMed] [Google Scholar]
- 20.Freeman, J. A., and B. L. Bassler. 1999. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 181:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman, J. A., B. N. Lilley, and B. L. Bassler. 2000. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol. Microbiol. 35:139-149. [DOI] [PubMed] [Google Scholar]
- 22.Fresenius, W., K. E. Quentin, and W. Schneider (ed.). 1988. Water analysis: a practical guide to physico-chemical, chemical and microbiological water examination and quality assurance, p. 420-421. Springer-Verlag, New York, N.Y.
- 23.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 24.Georgellis, D., O. Kwon, and E. C. Lin. 1999. Amplification of signaling activity of the arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J. Biol. Chem. 274:35950-35954. [DOI] [PubMed] [Google Scholar]
- 25.Gomez, J. A., M. T. Criado, and C. M. Ferreiros. 1998. Cooperation between the components of the meningococcal transferrin receptor, TbpA and TbpB, in the uptake of transferrin iron by the 37-kDa ferric-binding protein (FpbA). Res. Microbiol. 149:381-387. [DOI] [PubMed] [Google Scholar]
- 26.Gouhlen, F., A. Hafezi, V.-J. Uitto, D. Hinode, R. Nakamura, D. Grenier, and D. Mayrand. 1998. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 66:5307-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graber, K. R., L. M. Smoot, and L. A. Actis. 1998. Expression of iron binding proteins and hemin binding activity in the dental pathogen Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 163:135-142. [DOI] [PubMed] [Google Scholar]
- 28.Guan, L. L., and K. Kamino. 2001. Bacterial response to siderophore and quorum-sensing chemical signals in the seawater microbial community. BMC Microbiol. 1:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan, L. L., H. Onuki, and K. Kamino. 2000. Bacterial growth stimulation with exogenous siderophore and synthetic N-acyl homoserine lactone autoinducers under iron-limited and low-nutrient conditions. Appl. Environ. Microbiol. 66:2797-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haygood, M. G., and K. H. Nealson. 1985. Mechanisms of iron regulation of luminescence in Vibrio fischeri. J. Bacteriol. 162:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakelian, D., J. D. Lear, E. T. Lally, and J. C. Tanaka. 1998. Characterization of Actinobacillus actinomycetemcomitans leukotoxin pore formation in HL60 cells. Biochim. Biophys. Acta 1406:175-187. [DOI] [PubMed] [Google Scholar]
- 32.Kato, T., K. Honma, A. Yamanaka, T. Miura, and K. Okuda. 2000. Heterogeneity in the immune response to serotype b LPS of Actinobacillus actinomycetemcomitans in inbred strains of mice. FEMS Immunol. Med. Microbiol. 28:67-70. [DOI] [PubMed] [Google Scholar]
- 33.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 34.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. S. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 35.LaLonde, G., J. F. Miller, L. S. Tompkins, and P. O'Hanley. 1989. Transformation of Actinobacillus pleuropneumoniae and analysis of R factors by electroporation. Am. J. Vet. Res. 50:1957-1960. [PubMed] [Google Scholar]
- 36.Lear, J. D., D. Karakelian, U. Furblur, E. T. Lally, and J. C. Tanaka. 2000. Conformational studies of Actinobacillus actinomycetemcomitans leukotoxin: partial denaturation enhances toxicity. Biochim. Biophys. Acta 1476:350-362. [DOI] [PubMed] [Google Scholar]
- 37.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the luxRI homologs cepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewenza, S., and P. A. Sokol. 2001. Regulation of ornibactin biosynthesis and N-acyl-l-homoserine lactone production by CepR in Burkholderia cepacia. J. Bacteriol. 183:2212-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lilley, B. N., and B. L. Bassler. 2000. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36:940-954. [DOI] [PubMed] [Google Scholar]
- 40.Lyon, W. R., J. C. Madden, J. C. Levin, J. L. Stein, and M. G. Caparon. 2001. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 42:145-157. [DOI] [PubMed] [Google Scholar]
- 41.Matsubara, M., S. I. Kitaoka, S. I. Takeda, and T. Mizuno. 2000. Tuning of porin expression under anaerobic growth conditions by his-to-asp cross phosphorelay through both the EnvZ-osmosensor and ArcB-anaerosensor in Escherichia coli. Genes Cells 5:555-569. [DOI] [PubMed] [Google Scholar]
- 42.Matsushika, A., and T. Mizuno. 1998. A dual-signaling mechanism mediated by the ArcB hybrid sensor kinase containing the histidine-containing phosphotransfer domain in Escherichia coli. J. Bacteriol. 180:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushika, A., and T. Mizuno. 2000. Characterization of three putative sub-domains in the signal input domain of the ArcB hybrid sensor in Escherichia coli. J. Biochem. 127:855-860. [DOI] [PubMed] [Google Scholar]
- 44.Nowalk, A. J., K. G. Vaughn, B. W. Day, S. B. Tencza, and T. A. Meitzner. 1997. Metal-dependent conformers of the periplasmic ferric ion binding protein. Biochemistry 36:13054-13059. [DOI] [PubMed] [Google Scholar]
- 45.Oger, P., K. S. Kim, R. L. Sackett, K. R. Piper, and S. K. Farrand. 1998. Octopine-type Ti plasmids code for mannopine-inducible dominant-negative allele of TraR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol. Microbiol. 27:277-288. [DOI] [PubMed] [Google Scholar]
- 46.Page, M. I., and E. O. King. 1966. Infection due to Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. N. Engl. J. Med. 275:181-188. [DOI] [PubMed] [Google Scholar]
- 47.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell to cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 50.Pike, R. N., J. Potempa, W. McGraw, H. T. Coetzer, and J. Travis. 1996. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J. Bacteriol. 178:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson, P. B., M. Lantz, T. Marchuka, K. S. Kornman, C. I. Trummel, and S. C. Holt. 1982. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J. Periodont. Res. 17:175-283. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 53.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 54.Schauder, S., and B. L. Bassler. 2001. The languages of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 55.Schauder, S., K. Showkat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 56.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 57.Shenker, B. J., T. McKay, S. Datar, M. Miller, R. Chowden, and D. R. Demuth. 1999. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing G2 arrest in human T cells. J. Immunol. 162:4773-4780. [PubMed] [Google Scholar]
- 58.Shenker, B. S., R. H. Hoffmaster, T. McKay, and D. R. Demuth. 2000. Expression of cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J. Immunol. 165:2612-2618. [DOI] [PubMed] [Google Scholar]
- 59.Shi, Y., D. B. Ratnayake, K. Okamoto, N. Abe, K. Yamamoto, and K. Hakayama. 1999. Genetic analysis of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp and hagA. J. Biol. Chem. 274:17955-17960. [DOI] [PubMed] [Google Scholar]
- 60.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion on enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 64.Storey, D. G., E. E. Ujack, H. R. Rabin, and I. Mitchell. 1998. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 66:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surette, M. G., and B. L. Bassler. 1999. Regulation of autoinducer production in Salmonella typhimurium. Mol. Microbiol. 31:585-595. [DOI] [PubMed] [Google Scholar]
- 67.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taga, M. E., J. L. Semmelhack, and B. L. Bassler. 2001. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol. Microbiol. 42:777-793. [DOI] [PubMed] [Google Scholar]
- 69.Willemsen, P. T., I. Vulto, M. Boxem, and J. de Graaff. 1997. Characterization of a periplasmic protein involved in iron utilization of Actinobacillus actinomycetemcomitans. J. Bacteriol. 179:4949-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. G. Holden, R. Linforth, K. A. Cornell, A. J. Taylor, P. J. Hill, and P. Williams. 2002. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909-922. [DOI] [PubMed] [Google Scholar]
- 71.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 72.Yaku, H., M. Kato, T. Hakoshima, M. Tsuzuki, and T. Mizuno. 1997. Interaction between CheY response regulator and the histidine-containing phosphotransfer (HPt) domain of the ArcB sensory kinase in Escherichia coli. FEBS Lett. 408:337-340. [DOI] [PubMed] [Google Scholar]
- 73.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]
- 74.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]