Abstract
Secretory human phospholipase A2 type IIA (PLA2-IIA) catalyzes the hydrolysis of the sn-2 ester bond in glycerolipids to produce fatty acids and lysolipids. The enzyme is coupled to the inflammatory response, and its specificity toward anionic membrane interfaces suggests a role as a bactericidal agent. PLA2-IIA may also target perturbed native cell membranes that expose anionic lipids to the extracellular face. However, anionic lipid contents in native cells appear lower than the threshold levels necessary for activation. By using phosphatidylcholine/phosphatidylglycerol model systems, we show that local enrichment of anionic lipids into fluid domains triggers PLA2-IIA activity. In addition, the compositional range of enzyme activity is shown to be related to the underlying lipid phase diagram. A comparison is done between PLA2-IIA and snake venom PLA2, which in contrast to PLA2-IIA hydrolyzes both anionic and zwitterionic membranes. In general, this work shows that PLA2-IIA activation can be accomplished through local enrichment of anionic lipids into domains, indicating a mechanism for PLA2-IIA to target perturbed native membranes with low global anionic lipid contents. The results also show that the underlying lipid phase diagram, which determines the lipid composition at a local level, can be used to predict PLA2-IIA activity.
INTRODUCTION
The phospholipase A2 (PLA2) subtypes comprise a diverse family of enzymes that catalyze the hydrolysis of the sn-2 ester bond in glycerophospholipids, yielding free fatty acids and lysophospholipids (1). The first nonpancreatic mammalian PLA2 to be reported (2,3) was a 14-kDa calcium-dependent secretory PLA2 species, classified under the IIA group. PLA2-IIA is closely related to the inflammatory process (4), being involved in many acute and chronic inflammatory diseases. Large quantities of the enzyme were found in the synovial fluids in inflammatory arthritis (5). The enzyme is involved in the production of arachidonic acid (6), a precursor of bioactive eicosanoids. The enzyme has been found to be secreted by inflammatory effectors such as macrophages and mast cells (7–9), and the expression of the enzyme is regulated by proinflammatory cytokines (10). PLA2-IIA concentrations increase drastically in serum for a variety of inflammatory disorders, and high local levels of the enzyme are measured in inflamed tissues (9,11). PLA2-IIA has also been found to be overexpressed in the environment surrounding malignant tumors, and has been linked to increased aggressiveness of the tumor (12,13).
One of the distinguishing characteristics of PLA2-IIA is its specificity toward anionic membranes (14,15). The enzyme's activity toward neutral membranes composed of zwitterionic lipids such as phosphocholines is practically nonexistent, being several orders of magnitude lower than its activity toward anionic membranes (16). The specificity of the enzyme toward anionic membranes is a result of the electrostatic nature of the binding interactions between the enzyme and the surface (14,15,17). The enzyme has a relatively large patch of cationic residues at the surface that provide nonspecific electrostatic interactions, which promote binding to the anionic surface (15). Conversely, other PLA2 types, such as those isolated from snake venom, lack the large number of cationic residues found in PLA2-IIA (18). These species are able to bind and hydrolyze both zwitterionic and anionic lipid membranes, and do not exhibit the specificity of the PLA2-IIA (19). The lack of activity of PLA2-IIA toward zwitterionic membranes has been explained by the absence of a key tryptophan found in the snake venom PLA2 species, which is essential for the nonpolar interaction with the zwitterionic membrane (17). The specificity of PLA2-IIA has important physiological consequences. The outer leaflet of the plasma membrane of unperturbed mammalian cells are characterized by a neutral lipid composition enriched in sphingomyelin, phosphatidylcholines, and cholesterol (20). Unperturbed cells are therefore poor substrates for extracellular PLA2-IIA (14), and this is likely to prevent indiscriminate hydrolysis of healthy cells during PLA2-IIA upregulation.
Although its function during the inflammatory process is under discussion, the specificity of PLA2-IIA toward anionic interfaces points to its role as an antibacterial agent (21). PLA2-IIA is found to penetrate the highly anionic peptidoglycan bacterial cell wall, and hydrolyze to a large extent the phosphatidylglycerol lipids found at the bacterial membrane surface (21). This antimicrobial role appears to explain the high levels of PLA2-IIA found in tear fluid (22–24), and the close relationship between PLA2-IIA levels and the inflammatory response, which is normally triggered in the vicinity of wound sites. However, other lines of evidence also suggest that perturbed native cell membranes become vulnerable to PLA2-IIA hydrolysis. Apoptotic cells, for example, expose anionic lipids to the outer leaflet as a result of loss in membrane asymmetry, resulting in an anionic membrane surface prone to PLA2-IIA activity (25).
It has been pointed out that, after reaching equilibration across the bilayer during loss in membrane asymmetry, anionic lipid contents are not sufficiently high to induce binding of PLA2-IIA to the membrane surface (14), and that other factors must play a role in enhancing PLA2-IIA activity on perturbed cell systems. Some of the factors that have been suggested include an increase in phophatidic acid levels (14).
In this article, we investigate how membrane heterogeneity and domain formation can play a role in enhancing PLA2-IIA activity. Previous work has shown that snake venom PLA2 species are sensitive to membrane heterogeneity (26–28). Several studies have demonstrated that snake venom PLA2 exhibits increased activity in the presence of lipid domains. In particular, in a one-component dipalmitoylphosphatidylcholine (DPPC) system the lag time for snake venom PLA2, which is a latency period of low hydrolysis observed before a sudden burst in activity, reaches a minimum in the vicinity of the main phase-transition temperature (29,30). In a more physiologically relevant study, erythrocyte membranes were shown to become more vulnerable to hydrolysis by snake venom PLA2 as a result of an increase in membrane heterogeneity induced by an influx of Ca2+ (31–33). This increase in activity of snake venom PLA2 in the presence of a domain structure has been attributed to the formation of structural defects at the domain interface, which provide better access of the enzyme to its substrate (29).
Still unexplored are the questions of how lipid domains may regulate the activity of PLA2-IIA, and what similarities can be drawn from the snake venom PLA2 results. In particular, it is necessary to explore how the specificity of PLA2-IIA toward anionic membrane surfaces couples with the potential for lipid domains to regulate PLA2-IIA activity. In this article, we investigate these aspects using simple binary model systems and find that domain formation can act as an enhancer of PLA2-IIA activity in membranes with low anionic lipid content. However, in contrast to the snake venom PLA2 results, where enhanced PLA2 activity is a result of structural defects in the gel-fluid interface, the increased activity of PLA2-IIA in the presence of domains is induced by compositional heterogeneity and segregation of anionic lipids in the membrane surface. More specifically, domain formation induced by lipid phase separation results in regions enriched in anionic lipids, which trigger PLA2-IIA activity. The results described here therefore open the possibility that, in perturbed cell membranes presenting low anionic lipid contents in the outer leaflet, activation of PLA2-IIA can be induced through the formation of fluid-phase lipid domains enriched in anionic lipids.
MATERIALS AND METHODS
1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dimyristoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DMPG, a sodium salt), 1,2-distearoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DSPG, a sodium salt), and 1,2-di-O-octadecyl-sn-glycero-3-phosphocholine (1,2-di-O-SPC, nonhydrolizable ether lipid for calcein encapsulation) were purchased from Avanti Polar Lipids (Alabaster, AL) and were used without further purification. Calcein (2,4-bis-(N,N′-di(carboxymethyl)aminomethyl)-fluorescein) was purchased from ICN Biochemicals (Costa Mesa, CA). Sephadex G-50 was purchased from Pharmacia (Uppsala, Sweden). The specific PLA2-IIA inhibitor LY311727 was kindly provided by Eli Lilly (Indianapolis, IN). Tear fluid was used as the source for human PLA2-IIA. Tear fluid was collected from five healthy volunteers exposed to onion fumes and was pooled together. For the hydrolysis experiments, 10 μl of tear fluid was added for each aliquot. Tear fluid has a high concentration of PLA2-IIA and is the only prevalent PLA2 species found in tears. The PLA2-IIA content in tears in healthy subjects (54.5 μg/ml) is one of the highest amounts of PLA2-IIA content reported in human secretions (22–24). Purified snake venom PLA2 was a generous gift from Dr. R. L. Biltonen (University of Virginia, Charlottesville, VA). This PLA2 enzyme belongs to the class of low-molecular-weight 14-kDa secretory enzymes that display the capability of hydrolyzing both anionic and zwitterionic membranes (18).
Preparation of liposomes
Phosphoglycerol (PG) and phosphocholine (PC) liposomes
Appropriate amounts of lipid were dissolved and mixed in CHCl3/MeOH 9:1, placed under a stream of nitrogen gas, and dried under vacuum overnight. The dried lipids were dispersed in HEPES hydrolysis buffer (150 mM KCl, 10 mM HEPES, 30 μM CaCl2, 10 μM EDTA, pH 7.5) to a final concentration of 10 mM. Aqueous multilamellar lipid dispersions were prepared by repeated heating to 65°C, followed by vortexing for 15 min. The multilamellar vesicles were extruded using a Lipex Biomembranes (Vancouver, Canada) extruder 10 times through two stacked 100-nm-pore size polycarbonate filters (Whatman, Clifton, NJ), forming large unilamellar vesicles with a narrow size distribution. The extruder temperature was regulated using a water bath and was set to 65°C for all samples.
Calcein-enclosed 1,2-di-O-SPC liposomes
An appropriate amount of calcein (50 mM final concentration before adding to lipids) was dissolved in 5 ml of 1 M NaOH. After the calcein was fully dissolved, 2.5 ml of HEPES KCl-adjusted buffer (1 M KCl, 100 mM HEPES, 300 μM CaCl2, 100 μM EDTA, pH 7.5) was added together with 5 ml of MilliQ water (Millipore, Bedford, MA). The solution was then pH-adjusted to 7.5 with 1 M HCl dropwise to avoid precipitation. MilliQ water was then added to bring the calcein buffer to a final volume of 25 ml. 1,2-di-O-SPC unilamellar vesicles were prepared in the same manner as the phosphoglycerol (PG) and phosphocholine (PC) vesicles using the calcein buffer instead of the HEPES hydrolysis buffer during the lipid hydration step. Untrapped calcein was removed from the liposome suspension by gel filtration through a column packed with Sephadex G-50 using the HEPES hydrolysis buffer as an eluent. The vesicles are diluted 10-fold before being used in the activity assay. After 100% release, the fluorescence intensity of calcein as a function of concentration is still within the linear response regime (34).
Measurement of PLA2 activity through indirect calcein release from 1,2-di-O-SPC large unilamellar vesicles
Measurement of the activity of PLA2 on different PG/PC mixtures at different temperatures and phase states required an assay that would be insensitive both to temperature and to the phase behavior of the target liposomes. For this reason we decided to measure PLA2 activity by monitoring the release of calcein encapsulated in a self-quenching concentration (50 mM) from 1,2-di-O-SPC large unilamellar vesicles (100 nm). The 1,2-di-O-SPC vesicles are not hydrolyzed by PLA2, but, when added together with the target PG/PC vesicles, the release of their calcein content is triggered by the PG/PC hydrolysis products (34). The 1,2-di-O-SPC vesicles have a high phase-transition temperature (Tm=56°C). Therefore, within our experimental temperature range they are in a stable nonpermeable gel-phase regime. We observed no calcein release from the 1,2-di-O-SPC vesicles between 10 and 60°C (data not shown). For every data point, 37 μl of the PG/PC vesicles (10 mM) were added together with 25 μl of calcein containing 1,2-di-O-SPC vesicles into 2.4 ml of HEPES hydrolysis buffer within a cuvette. The cuvette was placed in a temperature-regulated cuvette holder within the fluorometer. Fluorescence measurements were performed using an SLM DMX 1100 spectrofluorometer (SLM Instruments, Urbana, IL). Excitation and emission channels were set at 490 nm and 515 nm, respectively, and the emission fluorescence was recorded as a function of time. An initial baseline fluorescence level was recorded in the first 50 s. PLA2 was added at this point, and calcein release was monitored. For each experimental point, 10 μl of tear fluid was used to monitor the human PLA2-IIA activity, whereas 4.4 μl of snake venom PLA2 (42 μM) was used to monitor the snake venom enzyme activity. Complete (100%) release was obtained by adding 50 μl of 10% Triton X-100 either after a plateau release level was obtained or after 1500 s in which no activity was observed.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed using a MicroCal MC-2 (Northhampton, MA) on samples of 10 mM DMPC/DMPG multilamellar vesicles at a scan rate of 10°C/h. An appropriate baseline was subtracted from the resulting thermograms. The onset and completion temperatures used to compose the DMPC/DSPG phase diagram were determined by intersecting the slope line at half-width on the peak heat capacity and the baseline (35).
RESULTS AND DISCUSSION
Evaluating PLA2-IIA and snake venom PLA2 activity through an indirect calcein release method
We were interested in evaluating the activity of PLA2 in a time-dependent manner for a variety of temperatures, membrane compositions, and phase states. For this reason we needed a reporter system that was relatively insensitive to both temperature and membrane phase behavior. An indirect calcein release method was found to be the most appropriate system (34). In this method, calcein-containing vesicles composed of nonhydrolyzable 1,2-di-O-SPC ether lipids are cosuspended with the PLA2 targeted vesicles. As the targeted vesicles become hydrolyzed, the products of hydrolysis diffuse into the nonhydrolyzable vesicles and trigger calcein release (Fig. 1 a). The 1,2-di-O-SPC vesicles are stable in a broad temperature regime. They have a gel/fluid phase-transition temperature (Tm = 56°C, as measured by DSC) that is higher than the temperature range to be probed, and they show no passive calcein release in the temperature range between 10 and 55°C (data not shown). As a result, the calcein-containing vesicles are insensitive to changes in temperature, are independent of the changes in composition of the PLA2 targeted vesicles, and only become permeable through the byproducts of the hydrolysis of the cosuspended target vesicles.
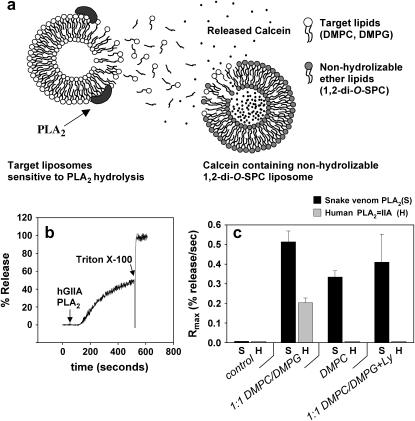
FIGURE 1.
(a) Detection of PLA2-IIA activity through release of calcein from nonhydrolyzable 1,2-di-O-SPC vesicles containing self-quenching concentrations of the fluorophore. The release is induced by migration of products of hydrolysis from the PLA2-IIA targeted vesicles, making the detection setup sensitive to PLA2-IIA activity, but independent of membrane phase behavior of the target system. (b) The activity of PLA2-IIA is assessed by monitoring calcein release as a function of time. PLA2-IIA is added at 50 s. Triton X-100 is added after the burst to induce total calcein release. The maximum calcein release rate (Rmax) is calculated by taking the maximum value in the first derivative of the activity trace. (c) Snake venom PLA2 and human PLA2-IIA activity are assessed for different experimental conditions specified at the column axis. All columns have the reporter 1,2-di-O-SPC vesicles present. The fourth column-set shows the activity of the enzymes in the presence of a PLA2-IIA inhibitor (Ly). See text for details.
Fig. 1 b shows a representative measurement of PLA2-IIA activity on 1:1 DMPC/DMPG unilamellar vesicles at 25°C. The enzyme is added at 50 s, and after a lag period of ∼70 s, a sharp increase in the intensity of the calcein fluorescence signal is observed, indicating the reaction burst. After the PLA2 burst Triton X-100 is added to induce total release. As a measure of enzyme activity, we calculated the maximum calcein release rate (Rmax), defined here as the inflection point in the reaction time courses, by taking the maximum value in the first derivative of the data. In Fig. 1 c, we present a series of control measurements at 25°C, in which the activities of PLA2-IIA and snake venom PLA2 are compared. To check the reliability of our enzyme source and experimental method we performed the following control measurements to corroborate that 1), calcein release unrelated to the PLA2 hydrolysis process is not observed after addition of PLA2-IIA; 2), hydrolysis is indeed measurable for PLA2-IIA for anionic lipid substrates; 3), PLA2-IIA shows specificity toward anionic lipid membranes; and 4), no PLA2-IIA activity is observed after treatment with a specific inhibitor. Nonhydrolyzable 1,2-di-O-SPC vesicles are added in every experimental condition in Fig. 1 c. The first set of columns (control) shows no enzyme activity for either the snake venom or the human PLA2 species in the absence of target vesicles, demonstrating that addition of either enzyme does not cause nonspecific release from the 1,2-di-O-SPC vesicles. In the next set of columns, both the snake venom PLA2 and human PLA2-IIA show activity toward anionic 1:1 DMPC/DMPG vesicles, with the snake venom PLA2 showing a higher degree of activity compared to PLA2-IIA for the enzyme concentrations used (see Materials and Methods). In the third set of columns, we observe that in the presence of zwitterionic DMPC vesicles only the snake venom enzyme is active, corroborating the specificity of PLA2-IIA toward anionic membranes. In the last set of columns, a specific PLA2-IIA inhibitor is added. Total inhibition is observed for the human PLA2-IIA. Partial inhibition is observed in the case of snake venom PLA2, which is expected for the type of inhibitor used.
Increased activity of PLA2-IIA in the fluid-phase regime for membranes enriched in anionic lipids
Extensive work has shown that snake venom PLA2 exhibits increased activity in the presence of gel/fluid domain coexistence (26–28). We were interested in determining whether PLA2-IIA exhibits a similar sensitivity toward gel/fluid interfaces. To evaluate this in a model system, we chose a 1:1 DMPC/DMPG mixture (Fig. 2 a). Both lipids have the same phase-transition temperature around 24°C (as measured by DSC) so the system is expected to behave as an ideal mixture, exhibiting a narrow transition from gel to fluid phase. However, as in the case of pure DPPC, the 1:1 DMPC/DMPG system is also expected to exhibit fluid/gel domain coexistence in the vicinity of 24°C. Saturated PC/PG mixtures are well characterized thermodynamically so they constitute a well defined setting for a quantitative study. We do not use 100% DMPG because the lipid is known to form nonvesicular three-dimensional structures at the main phase transition (36), which may restrict access to the enzyme.
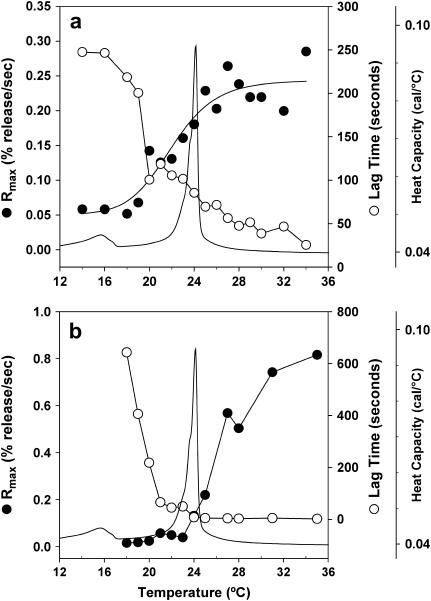
FIGURE 2.
(a) PLA2-IIA activity, and (b) snake venom PLA2 activity toward 1:1 DMPG/DMPC small unilamellar vesicles are assessed by plotting the maximum calcein release rate Rmax (•), and the lag time (○) as a function of temperature. The thermotropic phase behavior as determined by the heat capacity of 1:1 DMPG/DMPC multilamellar vesicles is also plotted in a and b to denote the gel/fluid phase transition of the system.
Fig. 2 a shows the main phase transition of 1:1 DMPC/DMPG vesicles at 24°C as measured by differential scanning calorimetry (a pretransition at 16°C indicates ripple phase formation). PLA2-IIA activity on 1:1 DMPC/DMPG vesicles was measured using the indirect calcein release assay. The lag time and the Rmax are also plotted as a function of temperature in Fig. 2 a. In contrast to previous results on snake venom PLA2 activity toward zwitterionic DPPC vesicles (29), neither a maximum in the activity nor a minimum in the lag time are observed at the main phase transition of the system. Instead, there is an increase in the activity, as measured by the release rate, and a decrease in the lag time as the system enters the fluid-phase temperature regime. This implies that, at least for the anionic/zwitterionic mixed system, PLA2-IIA does not rely on gel/fluid heterogeneities to access the substrate. The enzyme is therefore able to hydrolyze the negatively charged membrane in the fluid-phase regime.
The ability of PLA2-IIA to hydrolyze the fluid phase of the 1:1 DMPC/DMPG system in the absence of a gel/fluid domain structure could be a specific characteristic of PLA2-IIA that distinguishes the enzyme from the snake venom species. To test this, we evaluated the activity of snake venom PLA2 on the anionic/zwitterionic 1:1 DMPC/DMPG system. Fig. 2 b shows that snake venom PLA2 also exhibits increased activity in the fluid-phase regime of the 1:1 DMPC/DMPG system relative to the gel phase, also in contrast to what was found for the activity of snake venom PLA2 on DPPC bilayers (29). The increased activity of PLA2-IIA in the fluid-phase regime is therefore not an intrinsic property of the enzyme, but is a result of the properties of the anionic enriched substrate, which provides access to the enzyme in the fluid-phase regime. We rule out that the shorter chain length of the DMPC/DMPG system compared to the DPPC system induces this difference in behavior, since the same result as in Fig. 2 is obtained for the DPPC/DPPG system (data not shown). The increased activity of PLA2-IIA in the fluid-phase regime is then likely to be due either to the presence of the charged lipid that increases access to the substrate, or to heterogeneities in the fluid phase due to the presence of two distinct headgroups. In either case, the result does influence our understanding of the activation of PLA2-IIA in the presence of anionic lipids, since the enzyme does not necessitate the presence of a fluid/gel interface for activation. In addition, the model system we use to evaluate PLA2-IIA activity probably more closely resembles the situation in a cell membrane, where one would expect heterogeneous lipid composition to be present in the fluid phase. The result does not contradict earlier findings with snake venom PLA2 on zwitterionic systems, where the enzyme is indeed activated by fluid/gel domain formation. It is the presence of the anionic DMPG species that induces the increased activity in the fluid phase observed in Fig. 2.
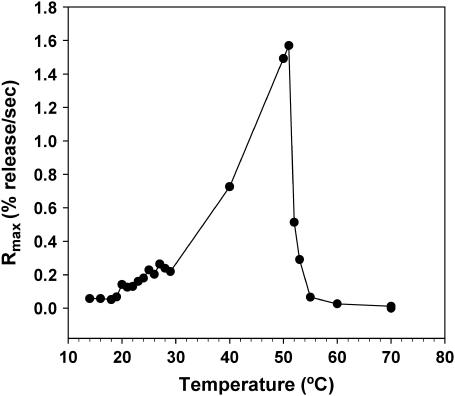
Fig. 3 shows that the activity of PLA2-IIA continues to increase with temperature up to 52°C, where a sudden drop in activity is observed. This sudden drop in enzyme activity above 52°C is reproducible in other systems, including the 1:1 DPPC/DPPG vesicles (data not shown). We therefore attribute this to thermal denaturation of the human PLA2-IIA enzyme. The enzyme behavior between 27 and 50°C is attributed to a thermally induced increase in enzyme activity. A plot of the logarithm of the calcein release rate versus the inverse in temperature appears linear between 30 and 50°C (data not shown), suggesting thermal activation of the enzyme. However, it should be noted that the fluorescence assay is not a direct measure of enzyme activity; therefore, this result is only suggestive and not strictly quantitative of a thermal increase in enzyme activity between 30 and 50°C. From Figs. 2 and 3 it is clear that PLA2-IIA shows increased activity throughout the fluid-phase regime in membranes enriched in anionic DMPG.
FIGURE 3.
Detecting the denaturation temperature for PLA2-IIA by monitoring the loss of enzyme activity for 1:1 DMPG/DMPC unilamellar vesicles as a function of temperature. PLA2-IIA activity is assessed by plotting maximum calcein release rates (Rmax) as a function of temperature.
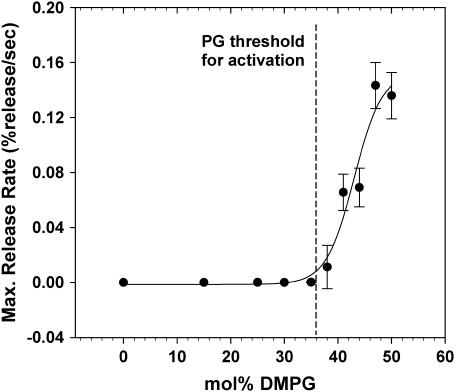
Fig. 4 shows a plot of the activity of PLA2-IIA as a function of DMPG percentage in DMPC/DMPG vesicles in the fluid-phase regime at 30°C. The plot shows the sigmoidal increase in activity with anionic lipid content expected for the interaction between the positively charged PLA2-IIA interface and the anionic membrane surface. The DMPG threshold concentration necessary for activation of PLA2-IIA is then measured to be ∼38 mol % DMPG. This threshold is higher than that reported for model systems using phosphatidylserine as the anionic species (14), which could be due to differences in the exposure of the hydrolysis site between the two headgroups. However, this needs to be investigated further.
FIGURE 4.
PLA2-IIA activity as a function of composition assessed by plotting maximum calcein release rates (Rmax) as a function of DMPG mol % in DMPG/DMPC mixtures at 30°C.
Activation of PLA2-IIA in the presence of fluid-phase lipid domains enriched in anionic lipids
In Fig. 2, it was shown that the presence of a fluid/gel domain interface does not enhance the activity of PLA2-IIA, at least in comparison to the activity of the enzyme toward fluid-phase membranes enriched in anionic lipids. This result appears to indicate that the structural heterogeneity that arose from gel/fluid domain formation may not enhance the activity of PLA2-IIA. However, we propose that domain formation can play a role in enhancing PLA2-IIA activity in the regime with lower anionic content by providing compositional heterogeneity. Based on the results from Fig. 2, which show that PLA2-IIA exhibits high activity toward anionic fluid-phase membranes, we focus on how the formation of fluid-phase domains may trigger the activation of PLA2-IIA through local enrichment with anionic lipids induced by lipid phase separation.
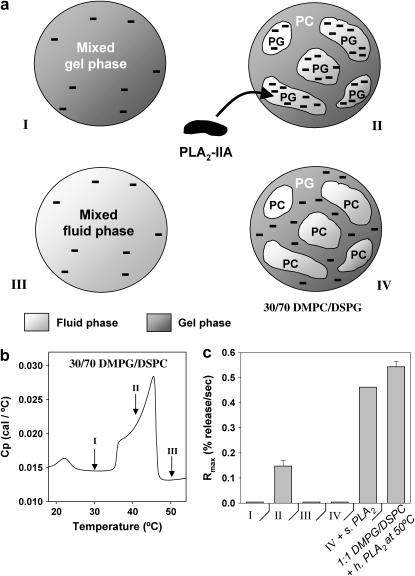
To investigate this proposal we chose a simple 30:70 DMPG/DSPC system, which presents a broad fluid/gel coexistence regime characterized by DMPG-enriched anionic fluid-phase domains and DSPC-enriched zwitterionic gel phase domains. Although the domain size should be dependent on the equilibration time, the composition of the domains is clearly defined by the phase boundaries. The overall DMPG content of this system is lower than the DMPG threshold concentration of 38 mol % presented in Fig. 4, so no activity is expected when the anionic lipid is evenly dispersed in the membrane. Fig. 5 b presents a DSC scan of 30:70 DMPG/DSPC multilamellar vesicles. The broad heat capacity profile between 35°C and 46°C indicates the main melting event of the system and demarcates the region of fluid/gel phase-domain coexistence. The lower temperature event at 22°C is the pretransition indicating the formation of the ripple phase. The domain structure within the main melting region (between 35°C and 46°C) is characterized by the presence of fluid-phase domains enriched in the lower melting component (negatively charged DMPG in this case), and gel-phase domains enriched in the higher melting component (zwitterionic DSPC) (37). We have indicated three different temperature points in the DSC trace (Fig. 5 b, I–III), which we evaluate with respect to PLA2-IIA activity. Region I is below the main melting event and is characterized by the membrane being fully in gel phase. Region II is characterized by the presence of fluid-phase domains enriched in DMPG (therefore highly anionic) and gel-phase domains enriched in DSPC. Region III is above the main melting event and is characterized by the membrane being fully in the fluid phase, and by having the anionic lipids evenly dispersed in the membrane. The three situations are represented by a cartoon in Fig. 5 a.
FIGURE 5.
(a) Cartoon of unilamellar vesicles presenting the following phase states and membrane domain structures: (I) all gel phase at 30°C; (II) coexisting fluid and gel phase domains at 40°C, where the fluid-phase domains are enriched in the anionic lipid DMPG; (III) all fluid phase at 50°C; and (IV) coexisting fluid and gel phase domains where the fluid-phase domains are enriched in the zwitterionic lipid DMPC. Regions I–III are from vesicles of the same composition, 30:70 DMPG/DSPC; in region IV, the composition is 30:70 DMPC/DSPG unilamellar vesicles. (b) Thermotropic phase behavior (heat capacity) of the 30:70 DMPG/DSPC samples showing the three phase-behavior scenarios (for regions I–III) as different points in the thermogram. (c) PLA2-IIA activity toward the four systems presented in a. The activity of snake venom PLA2 is assessed for region IV in the fifth column, and the activity of PLA2-IIA is assessed at 50°C for 1:1 DMPG/DSPC vesicles in the sixth column.
A 30:70 DMPC/DSPG system (IV) is also evaluated for PLA2-IIA activity, where the headgroups of the lower-melting-temperature component and the higher-melting-temperature component have been switched. This system has a gel/fluid coexistence temperature range similar to that of DMPG/DSPC, except that it is characterized by the presence of fluid-phase domains enriched in zwitterionic DMPC and gel-phase domains enriched in anionic DSPG. We expect low PLA2-IIA activity in this case, since the fluid-phase domains are enriched in a zwitterionic lipid (Fig. 5 a-IV).
In Fig. 5 c, the level of activity of PLA2-IIA toward the four systems is evaluated. PLA2-IIA shows no activity when the 30:70 DMPG/DSPC vesicles are in the gel-phase regime (I). It should be pointed out that not even a baseline level of activity is observed, in contrast to a small but significant level of activity previously observed (Fig. 2 a) in the gel phase for 1:1 DMPG/DMPC vesicles. This indicates that in addition to the reduced activity of PLA2-IIA toward the gel phase, the low DMPG level leads to a further reduction in activity. PLA2-IIA also shows low activity in the fluid-phase regime (III), where the DMPG lipids are expected to be evenly distributed in the membrane and the local concentration of DMPG is lower than the anionic lipid threshold. In contrast, PLA2-IIA shows significant activity in the presence of fluid/gel domain coexistence (II), where the fluid-phase domains are enriched in DMPG. This result shows that local enrichment in anionic lipids can overcome the anionic lipid threshold needed to trigger PLA2-IIA activity. No activity is observed in region IV, where the fluid-phase domains are enriched in a zwitterionic lipid (DMPC) and the gel-phase domains are enriched in the anionic lipid (DSPG), showing that it is specifically the fluid-phase domains enriched in anionic lipids that can trigger PLA2-IIA activity. As controls we tested the activity of snake venom PLA2 on the 30:70 DMPC/DSPG system (Fig. 5 c, fifth column). As expected, the snake venom enzyme shows activity in the presence of zwitterionic fluid-phase domains, in contrast to PLA2-IIA, which shows no activity toward the 30:70 DMPC/DSPG system (Fig. 5 c, fourth column). PLA2-IIA activity in 1:1 DMPG/DMPC at 50°C (Fig. 5 c, sixth column) has also been tested to make sure that the lack of activity in region III is not due to enzyme denaturation.
The DMPG/DSPC phase diagram predicts the windows of activity for PLA2-IIA
In Fig. 6, we compare the activity window of PLA2-IIA to the thermotropic behavior, as measured by DSC, for three different DMPG/DSPC compositions. Fig. 6 a shows the activity window of a 1:1 DMPG/DSPC system, which ranges from 22°C to 52°C. The gel-fluid coexistence regime, as determined by the 1:1 DMPG/DSPC thermogram, ranges from 28°C to 42°C. The DMPG content of this system is higher than the anionic lipid threshold concentration for activation; therefore, PLA2-IIA should show activity in the fluid-phase regime when DMPG is evenly distributed. As expected, PLA2-IIA activity continues increasing in an Arrhenius manner in the fluid-phase regime until the denaturation temperature of the enzyme at 52°C. In the lower temperature range the window of activity for PLA2-IIA extends unexpectedly below the gel/fluid coexistence regime. An explanation for this may be the presence of the ripple phase between 20°C and 28°C. Previously, we showed that the ripple phase enhances the activity of snake venom PLA2 in DMPC/DSPC mixtures compared to the gel phase (38,39). The increased local curvature due to the ripples may not in itself necessarily lead to an increase in PLA2-IIA activity, considering that we found that the gel/fluid interface does not enhance PLA2-IIA activity (Fig. 2 a). However, in addition it was found that snake venom PLA2 showed preferential hydrolysis of the lower melting component in the DMPC/DSPC system, which appeared to indicate a degree of phase separation in the ripple-phase regime (38,39). The increased activity of PLA2-IIA in the ripple-phase regime may also be related to a certain degree of phase separation between DMPG and DSPC.
FIGURE 6.
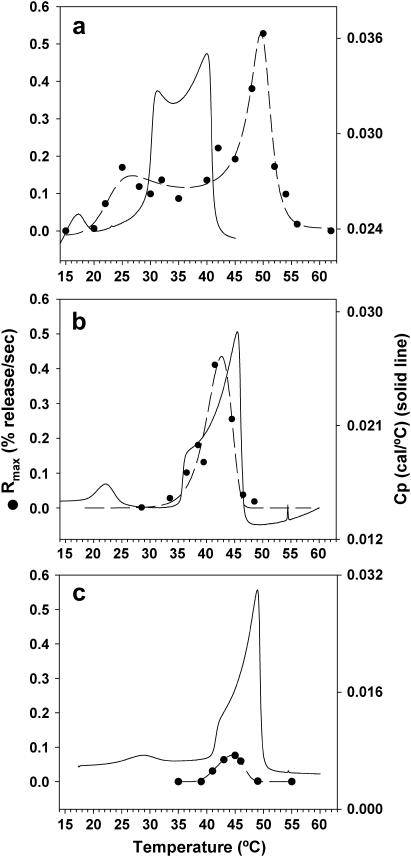
PLA2-IIA activity as a function of temperature for (a) 1:1 DMPG/DSPC, (b) 30:70 DMPG/DSPC, and (c) 20:80 DMPG/DSPC unilamellar vesicles. The thermotropic phase behavior as measured by DSC of each one of the systems is included to indicate the fluid/gel coexistence regimes.
Fig. 6 b presents the PLA2-IIA activity window for a 30:70 DMPG/DSPC system. It can be seen that the enzyme activity is restricted to the fluid/gel coexistence regime, where the fluid-phase domains are enriched in DMPG. In contrast to Fig. 6 a, no activity is observed in the fluid-phase regime for the 30:70 DMPG/DSPC system. In Fig. 6 c, we evaluate the activity window for the 20:80 DMPG/DSPC system. The activity of the enzyme is also restricted to the gel/fluid coexistence regime, and no activity is observed in the fluid-phase regime. The maximum activity in the 20:80 DMPG/DSPC system is significantly lower than in the 30:70 DMPG/DSPC system. This point is addressed later in the discussion of Fig. 7 b.
FIGURE 7.
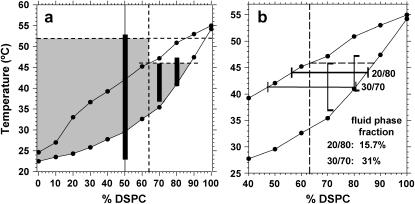
(a) PLA2-IIA activity in relation to the DMPG/DSPC phase diagram. The fluidus and solidus phase lines (•) were determined from the onset and completion temperatures for the corresponding DMPG/DSPC thermograms. Top horizontal dashed line represents the denaturation temperature for PLA2-IIA. Vertical dashed line represents the anionic lipid threshold for PLA2-IIA activity. Second horizontal dashed line is a tie line in the phase diagram where the fluid-phase domain composition corresponds to the anionic activation threshold of the enzyme. The black vertical bars represent the measured PLA2-IIA activity windows for three different compositions. (b) Tie lines corresponding to the temperature where maximum activity is observed for the 30:70 DMPG/DSPC and 20:80 DMPG/DSPC samples. The fluid-phase fractions calculated from the tie lines for each composition are shown in the lower right corner of the figure.
Fig. 7 a shows the DMPG/DSPC phase diagram as determined by DSC. The gel/fluid coexistence regime is defined by the solidus and fluidus phase lines (solid lines with solid circles), which are determined from the onset and completion temperatures of the main melting event in the DSC thermograms. The solidus (bottom) phase line corresponds to a cooperative transition from 100% gel phase to gel/fluid coexistence, whereas the fluidus (top) phase line corresponds to a cooperative transition from 100% fluid phase to gel/fluid coexistence. The additional lines that are drawn into the phase diagram are related to the enzyme activity. The top horizontal dashed line crossing at 52°C corresponds to the denaturation temperature of the enzyme, and the vertical dashed line crossing at 64 mol % DSPC corresponds to the DMPG threshold concentration for PLA2-IIA activation. In the absence of a fluid/gel domain structure, the rectangular region demarcated by these two dashed lines (T < 52°C; % DSPC < 64) would define the activity window of the enzyme.
However, due to the presence of fluid/gel domains originating in the phase diagram, an additional region of activity stretches into the low-DMPG-concentration regime (right-hand side of the phase diagram). This region of activity is expected to be delimited by the solidus phase line (where the anionic fluid-phase domains nucleate), and a higher temperature border determined by a tie-line in the phase diagram (second horizontal dashed line crossing at ∼46°C). This tie line corresponds to fluid-phase domains with DMPG content equal to the anionic lipid threshold. Above this tie line, the fluid-phase domains will have a DMPG content lower than the anionic lipid threshold.
The shaded area therefore corresponds to the region in the phase diagram where fluid-phase formation is expected, and where the fluid phase will have a DMPG concentration greater than the anionic lipid threshold for PLA2-IIA activation. The shaded area is also limited by the denaturation temperature of the enzyme. It therefore corresponds to the region in the phase diagram where PLA2-IIA activity is expected.
We have included the experimental data as three vertical bars in Fig. 7 a corresponding to the activity windows observed for the 50:50, 30:70, and 20:80 DMPG/DSPC vesicles as measured in Fig. 6. The length of the vertical bars was determined from Fig. 6, a–c, by taking the temperatures corresponding to an activity value of at least 20% of the maximum activity observed for the corresponding composition. We observe that the bars correspond closely to the shaded area in the higher-temperature border (Fig. 7 a). In the case of the 50:50 DMPG/DSPC sample the activity window extends by several °C into the ripple phase regime. As discussed previously, the activity in the ripple-phase regime may be explained by phase separation of a small fraction of the lower melting temperature component in the ripple structure (38,39); however, this needs further investigation to be corroborated. The bars corresponding to the lower anionic lipid concentrations at 30:70 and 20:80 fit more closely to the boundaries of the activity window.
An additional result that may be explained by the DMPG/DSPC phase diagram is the reduction in maximum activity observed in the 20:80 sample compared to the 30:70 sample. Fig. 7 b shows the two vertical activity windows corresponding to the two compositions. Tie lines have been drawn through the midpoint of each activity window. With the use of the lever rule, the fractions of fluid phase and gel phase corresponding to each composition at the midpoint of the activity window can be determined. It is clear that the percentage of fluid phase available for hydrolysis in the 20:80 sample is much smaller than in the 30:70 sample, which explains the fivefold reduction in activity observed in Fig. 6. This reduction in activity is explained by a reduction of the fluid-phase fraction available for hydrolysis.
CONCLUSION
In this work, we have shown that local enhancement of anionic lipid concentrations induced by domain formation can trigger PLA2-IIA activity. The formation of fluid-phase domains enriched in anionic lipids may explain, at least in part, the activation of PLA2-IIA toward perturbed cell membrane systems that have undergone loss in membrane asymmetry. The low global levels of anionic lipids in cell plasma membranes appear to be less than the anionic lipid content required for activation (14). However, the activity of PLA2-IIA during inflammatory response indicates that the enzyme is able to hydrolyze perturbed host cell systems (9,25). Plasma membranes have been shown to form lipid domain systems (40–42) through phase separation of liquid-ordered phase domains or lipid rafts, which are enriched in both cholesterol and high-melting-temperature components such as sphingomyelin (20). The formation of these cholesterol-enriched domains entails the presence of coexisting cholesterol-poor fluid-phase domains enriched in low-melting-temperature components. Anionic lipids in the cell plasma membrane, such as phosphatidylserine, are characterized by having unsaturated chains (43), and would therefore tend to prefer the fluid-phase domains. One of the targets of PLA2-IIA hydrolysis that plays an important role during the inflammatory response is arachadonic acid (25); a highly unsaturated fatty acid likely to induce its corresponding phospholipid to partition into the fluid-phase domains. The activation of PLA2-IIA toward perturbed cellular systems may be induced by the formation of lipid domains enriched in unsaturated anionic lipids.
To understand in more depth the behavior of PLA2-IIA in the presence of coexisting phases, it was necessary to embark on a fundamental study with a simple two-component domain system. This study opens the door for the analysis of PLA2-IIA activity in more complex mixtures that include cholesterol and reflect the composition of eukaryotic cell membranes. Although extensive work has shown that snake venom PLA2 presents an increase in activity in the presence of domain interface defects, no biophysical studies have been performed on the effects of lipid domain structures on the human enzyme. It is clear from this study that in the presence of membranes containing anionic lipids, human PLA2-IIA is strongly regulated by membrane composition and not by the presence of domain interface defects. This observation changes the perception of the factors that regulate PLA2-IIA activity in cell membranes, and it lays the groundwork for studying the activity of human PLA2-IIA in the presence of more complex domain systems.
This study has also shown that PLA2-IIA activity could be mapped onto a multi-component phase diagram where the domain fractions and compositions are well defined, and where the anionic lipid content of the fluid-phase domains can be determined based on the phase lines. In this way, the anionic lipid content in the fluid-phase domains can be clearly related to the anionic lipid threshold of the enzyme. The same strategy can be followed to evaluate the activity of the enzyme within more complex phase diagrams that might lead to a more detailed understanding of the phase behavior and domain structures found in perturbed cell membranes and their role in regulating activity. Three-component phase diagrams (1-palmitoyl, 2-oleoyl phosphatidylcholine-sphingomyelin-cholesterol), which reflect the phase behavior of cell membranes more closely, are becoming available (44–46). It would be necessary to include phosphatidylserine in the development of more complex phase diagrams to begin testing some predictions of the compositional regions where activation of human PLA2-IIA may be expected. It is likely that cholesterol will play a key role in regulating the nature of the domains present, and the possibility for activation of PLA2-IIA.
Acknowledgments
The authors thank Simon S. Jensen for his help in the use of the PLA2-IIA inhibitor.
MEMPHYS-Center for Biomembrane Physics is supported by the Danish National Research Foundation.
References
- 1.Six, D. A., and E. A. Dennis. 2000. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta. 1488:1–19. [DOI] [PubMed] [Google Scholar]
- 2.Kramer, R. M., C. Hession, B. Johansen, G. Hayes, P. Mcgray, E. P. Chow, R. Tizard, and R. B. Pepinsky. 1989. Structure and properties of a human non-pancreatic phosopholipase A2. J. Biol. Chem. 264:5768–5775. [PubMed] [Google Scholar]
- 3.Seilhamer, J. J., W. Pruzanski, P. Vadas, S. Plant, J. A. Miller, J. Kloss, and L. K. Johnson. 1989. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial-fluid. J. Biol. Chem. 264:5335–5338. [PubMed] [Google Scholar]
- 4.Nevalainen, T. J., M. M. Haapamaki, and J. M. Gronroos. 2000. Roles of secretory phospholipases A2 in inflammatory diseases and trauma. Biochim. Biophys. Acta. 1488:83–90. [DOI] [PubMed] [Google Scholar]
- 5.Pruzanski, W., P. Vadas, E. Stefanski, and M. B. Urowitz. 1985. Activity of phospholipase A2 in sera and synovial fluids in arthritis. J. Rheumatol. 12:211–216. [PubMed] [Google Scholar]
- 6.Murakami, M., S. Shimbara, T. Kambe, H. Kuwata, M. V. Winstead, J. A. Tischfield, and I. Kudo. 1998. The function of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 273:14411–14423. [DOI] [PubMed] [Google Scholar]
- 7.Kudo, I., M. Murakami, S. Hara, and K. Inoue. 1993. Mammalian non-pancreatic phospholipases A2. Biochim. Biophys. Acta. 1170:217–231. [DOI] [PubMed] [Google Scholar]
- 8.Murakami, M., I. Kudo, and K. Inoue. 1995. Secretory phospholipase A2. J. Lipid Mediat. Cell Signal. 12:119–130. [DOI] [PubMed] [Google Scholar]
- 9.Koike, K., Y. Yamamoto, H. Otsuka, M. Murakami, and I. Kudo. 1997. Phospholipase A2 in inflamed tissues and inflammatory exudates. In Phospholipase A2. Basic and Clinical Aspects in Inflammatory Disease. W. Uhl, T. J. Nevalainen, and M. W. Buchler, editors. Karger, Basel, Switzerland. 94–103.
- 10.Scholz, K., G. J. Vlachojannis, S. Spitzer, V. Schini-Kerth, H. van den Bosch, M. Kaszkin, and J. Pfeilschifter. 1999. Modulation of cytokine-induced expression of secretory phospholipase A2-type IIA by protein kinase C in rat renal mesangial cells. Biochem. Pharmacol. 58:1751–1758. [DOI] [PubMed] [Google Scholar]
- 11.Nevalainen, T., and J. M. Grönroos. 1997. Serum phospholipase A2 in inflammatory disease. In Phospholipase A2. Basic and Clinical Aspects in Inflammatory Disease. W. Uhl, T. J. Nevalainen, and M. W. Buchler, editors. Karger, Basel, Switzerland. 104–109.
- 12.Abe, T., K. Sakamoto, H. Kamohara, Y. Hirano, N. Kuwahara, and M. Ogawa. 1997. Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int. J. Cancer. 74:245–250. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa, M. 1997. Group II phospholipase A2 in neoplastic disease. In Phospholipase A2. Basic and Clinical Aspects in Inflammatory Disease. W. Uhl, T. J. Nevalainen, and M. W. Buchler, editors. Karger, Basel, Switzerland. 200–204.
- 14.Buckland, A. G., and D. C. Wilton. 2000. Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim. Biophys. Acta. 1483:199–216. [DOI] [PubMed] [Google Scholar]
- 15.Canaan, S., R. Nielsen, F. Ghomashchi, B. H. Robinson, and M. H. Gelb. 2002. Unusual mode of binding of human group IIA secreted phospholipase A2 to anionic interfaces as studied by continuous wave and time domain electron paramagnetic resonance spectroscopy. J. Biol. Chem. 277:30984–30990. [DOI] [PubMed] [Google Scholar]
- 16.Bezzine, S., R. S. Koduri, E. Valentin, M. Murakami, I. Kudo, F. Ghomashchi, M. Sadilek, G. Lambeau, and M. H. Gelb. 2000. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 275:3179–3191. [DOI] [PubMed] [Google Scholar]
- 17.Bezzine, S., J. G. Bollinger, A. G. Singer, S. L. Veatch, S. L. Keller, and M. H. Gelb. 2002. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277:48523–48534. [DOI] [PubMed] [Google Scholar]
- 18.Han, S. K., E. T. Yoon, D. L. Scott, P. B. Sigler, and W. Cho. 1997. Structural aspects of interfacial adsorption. A crystallographic and site-directed mutagenesis study of the phospholipase A2 from the venom of Agkistrodon piscivorus piscivorus. J. Biol. Chem. 272:3573–3582. [PubMed] [Google Scholar]
- 19.Gadd, M. E., and R. L. Biltonen. 2000. Characterization of the interaction of phospholipase A2 with phosphatidylcholine-phosphatidylglycerol mixed lipids. Biochemistry. 39:9623–9631. [DOI] [PubMed] [Google Scholar]
- 20.Silvius, J. R. 2003. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys. J. 85:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckland, A. G., and D. C. Wilton. 2000. The antibacterial properties of secreted phospholipases A2. Biochim. Biophys. Acta. 1488:71–82. [DOI] [PubMed] [Google Scholar]
- 22.Aho, V. V., J. M. Holopainen, T. Tervo, J. A. O. Moilanen, T. Nevalainen, and K. M. Saari. 2003. Group IIA phospholipase A2 content in tears of patients having photorefractive keratectomy. J. Cataract Refract. Surg. 29:2163–2167. [DOI] [PubMed] [Google Scholar]
- 23.Aho, V. V., T. J. Nevalainen, and K. M. Saari. 2002. Group IIA phospholipase A2 content of basal, nonstimulated and reflex tears. Curr. Eye Res. 24:224–227. [DOI] [PubMed] [Google Scholar]
- 24.Saari, K. M., V. V. Aho, V. Paavilainen, and T. J. Nevalainen. 2001. Group II PLA2 content of tears in normal subjects. Invest. Ophthalmol. Vis. Sci. 42:318–320. [PubMed] [Google Scholar]
- 25.Atsumi, G., M. Murakami, M. Tajima, S. Shimbara, N. Hara, and I. Kudo. 1997. The perturbed membrane of cells undergoing apoptosis is susceptible to type II secretory phospholipase A2 to liberate arachidonic acid. Biochim. Biophys. Acta. 1349:43–54. [DOI] [PubMed] [Google Scholar]
- 26.Burack, W. R., Q. Yuan, and R. L. Biltonen. 1993. Role of lateral phase-separation in the modulation of phospholipase-A2 activity. Biochemistry. 32:583–589. [DOI] [PubMed] [Google Scholar]
- 27.Burack, W. R., and R. L. Biltonen. 1994. Lipid bilayer heterogeneities and modulation of phospholipase A2 activity. Chem. Phys. Lipids. 73:209–222. [DOI] [PubMed] [Google Scholar]
- 28.Bell, J. D., M. Burnside, J. A. Owen, M. L. Royall, and M. L. Baker. 1996. Relationships between bilayer structure and phospholipase A2 activity: Interactions among temperature, diacylglycerol, lysolecithin, palmitic acid, and dipalmitoylphosphatidylcholine. Biochemistry. 35:4945–4955. [DOI] [PubMed] [Google Scholar]
- 29.Hønger, T., K. Jørgensen, R. L. Biltonen, and O. G. Mouritsen. 1996. Systematic relationship between phospholipase A2 activity and dynamic lipid bilayer microheterogeneity. Biochemistry. 35:9003–9006. [DOI] [PubMed] [Google Scholar]
- 30.Høyrup, P., O. G. Mouritsen, and K. Jørgensen. 2001. Phospholipase A2 activity towards vesicles of DPPC and DMPC-DSPC containing small amounts of SMPC. Biochim. Biophys. Acta. 1515:133–143. [DOI] [PubMed] [Google Scholar]
- 31.Smith, S. K., A. R. Farnbach, R. Amelia, F. H. Harris, M. Faith, A. C. Hawes, C. Andrea, L. R. Jackson, A. M. Judd, R. S. Vest, S. Sanchez, and J. D. Bell. 2001. Mechanisms by which intracellular calcium induces susceptibility to secretory phospholipase A2 in human erythrocytes. J. Biol. Chem. 276:22732–22741. [DOI] [PubMed] [Google Scholar]
- 32.Vest, R. S., L. J. Gonzales, S. A. Permann, E. Spencer, L. D. Hansen, A. M. Judd, and J. D. Bell. 2004. Divalent cations increase lipid order in erythrocytes and susceptibility to secretory phospholipase A2. Biophys. J. 86:2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen, L. B., N. K. Burgess, D. D. Gonda, E. Spencer, H. A. Wilson-Ashworth, E. Driscoll, M. P. Vu, J. L. Fairbourn, A. M. Judd, and J. D. Bell. 2005. Mechanisms governing the level of susceptibility of erythrocyte membranes to secretory phospholipase A2. Biophys. J. 88:2692–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidsen, J., K. Jørgensen, T. L. Andresen, and O. G. Mouritsen. 2003. Secreted phospholipase A2 as a new enzymatic trigger mechanism for localised liposomal drug release and absorption in diseased tissue. Biochim. Biophys. Acta. 1609:95–101. [DOI] [PubMed] [Google Scholar]
- 35.Mabrey, S., and J. M. Sturtevant. 1976. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 73:3862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider, M. F., D. Marsh, W. Jahn, B. Kloesgen, and T. Heimburg. 1999. Network formation of lipid membranes: triggering structural transitions by chain melting. Proc. Natl. Acad. Sci. USA. 96:14312–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leidy, C., W. F. Wolkers, K. Jørgensen, O. G. Mouritsen, and J. H. Crowe. 2001. Lateral organization and domain formation in a two-component lipid membrane system. Biophys. J. 80:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidy, C., O. G. Mouritsen, K. Jørgensen, and G. H. Peters. 2004. Evolution of a rippled membrane during phospholipase A2 hydrolysis studied by time-resolved AFM. Biophys. J. 87:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leidy, C., T. Kaasgaard, O. G. Mouritsen, K. Jørgensen, and G. H. Peters. 2005. Investigation of lipid membrane organization, morphology, and dynamics by atomic force microscopy of supported bilayers. In Recent Research Developments in Biophysics. S. G. Pandalai, editor. Transworld Research Network, Kerela, India. 163–186.
- 40.Vereb, G., J. Szollosi, J. Matko, P. Nagy, T. Farkas, L. Vigh, L. Matyus, T. A. Waldmann, and S. Damjanovich. 2003. Dynamic, yet structured: the cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. USA. 100:8053–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. [DOI] [PubMed] [Google Scholar]
- 42.Brown, R. E., W. H. Anderson, and V. S. Kulkarni. 1995. Macro-ripple phase formation in bilayers composed of galactosylceramide and phosphatidylcholine. Biophys. J. 68:1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, T. Y., and J. R. Silvius. 2001. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 81:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feigenson, G. W., and J. T. Buboltz. 2001. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys. J. 80:2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]