Abstract
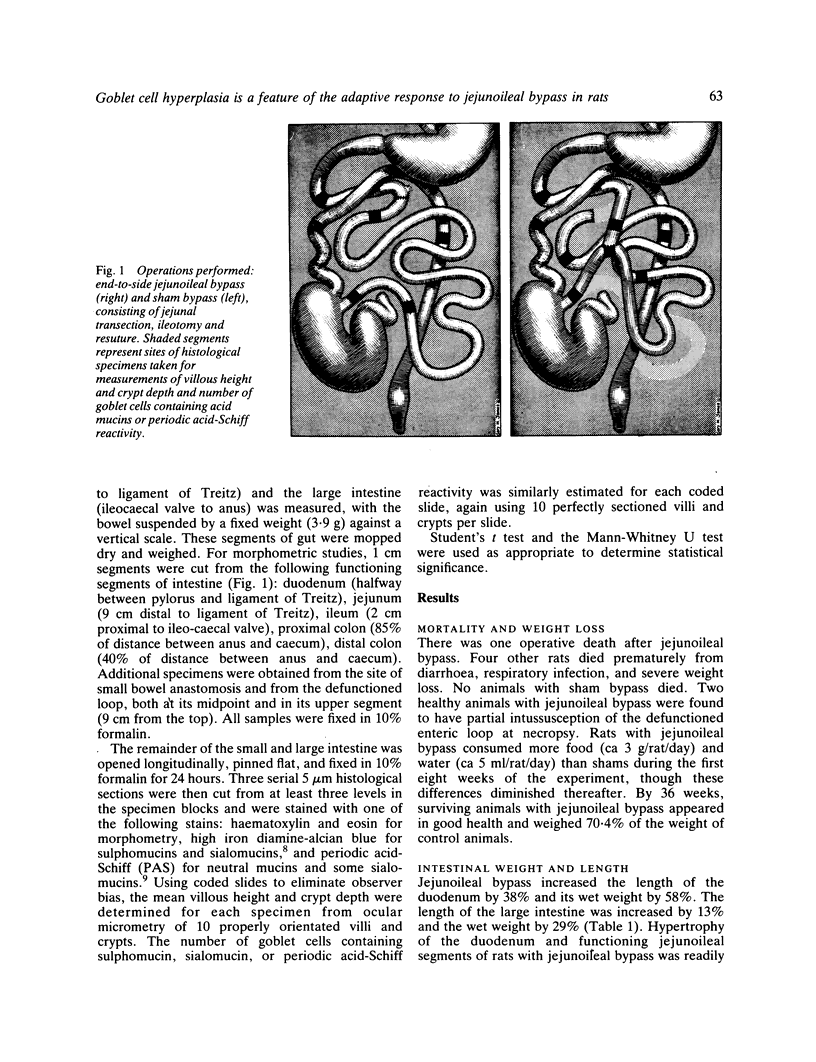
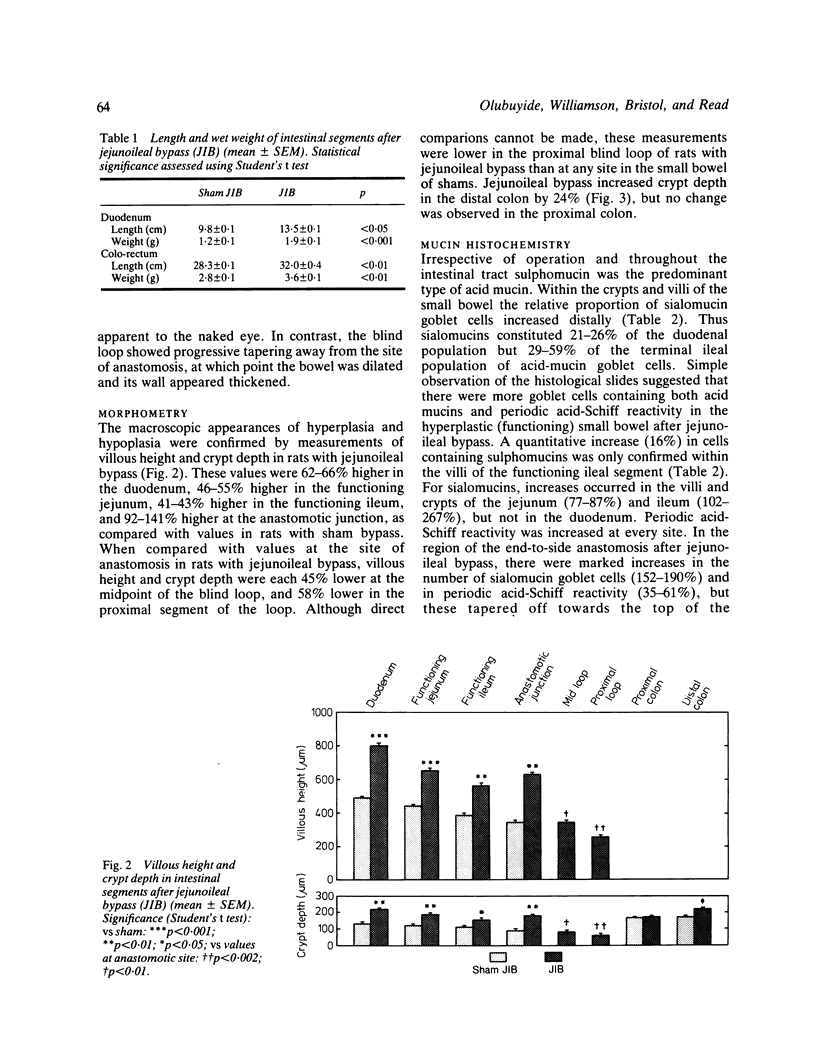
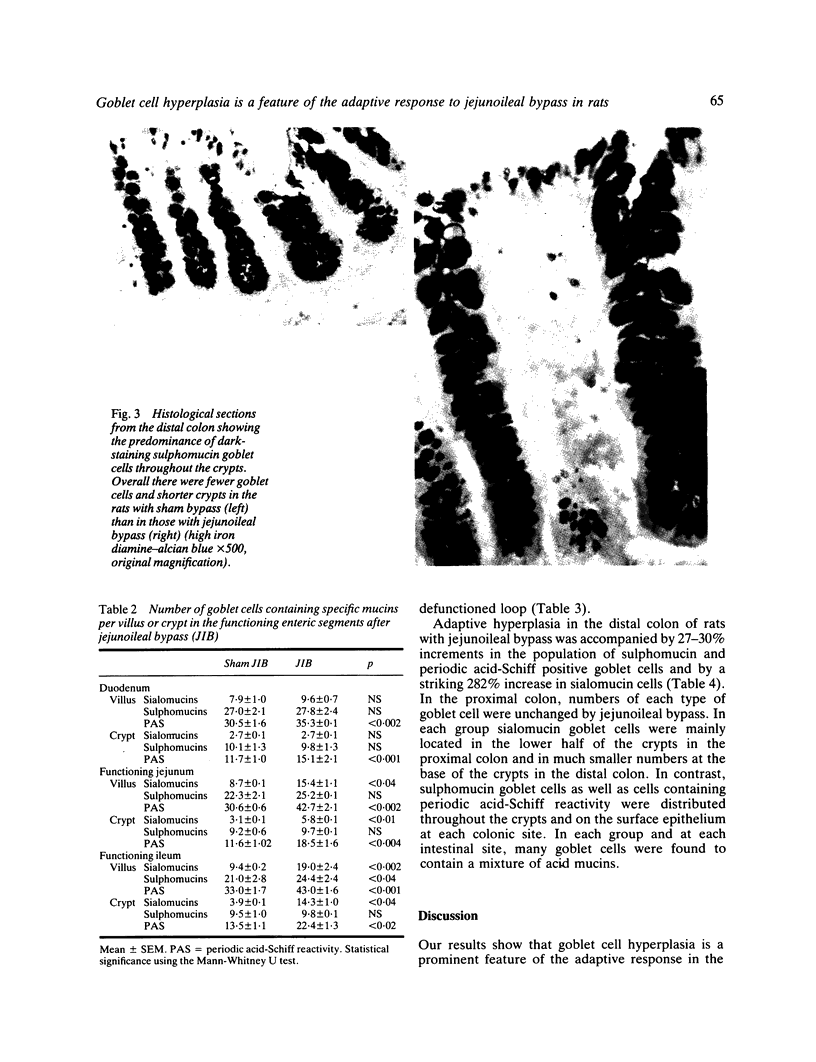
The role of goblet cells in the adaptive response of the intestine to jejunoileal bypass was studied in rats submitted to an 85% end-to-side jejunoileal bypass or sham bypass. At 36 weeks the length and wet weight of the duodenum and large bowel was 13-48% greater in animals with jejunoileal bypass. Measurements of villous height and crypt depth confirmed mucosal hyperplasia in the residual functioning small bowel and the distal colon. Histochemical studies in both groups of rats showed an overall predominance of sulphomucins throughout the intestinal tract, but jejunoileal bypass caused a disproportionate increase in the number of sialomucin containing goblet cells in functioning segments of small bowel and distal colon. An abundance of sialomucin cells at the site of anastomosis after jejunoileal bypass may have been a protective response to local mechanical trauma. Goblet cell hyperplasia is a feature of compensatory growth of the intestinal tract after surgical shortening. The changes in colonic mucin seen after jejunoileal bypass resemble those observed in ulcerative colitis and mucosal dysplasia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Mucin secretion in inflammatory bowel disease: correlation with disease activity and dysplasia. Gut. 1982 Jun;23(6):485–489. doi: 10.1136/gut.23.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Filipe M. I. Mucous secretion in rat colonic mucosa during carcinogenesis induced by dimethylhydrazine. A morphological and histochemical study. Br J Cancer. 1975 Jul;32(1):60–77. doi: 10.1038/bjc.1975.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R., Filipe M. I. Sulphomucins and precancerous lesions of the human stomach. Histopathology. 1980 May;4(3):271–279. doi: 10.1111/j.1365-2559.1980.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Joffe S. N. Surgical management of morbid obesity. Gut. 1981 Mar;22(3):242–254. doi: 10.1136/gut.22.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Litt M., Khan M. A., Wolf D. P. Mucus rheology: relation to structure and function. Biorheology. 1976 Feb;13(1):37–48. doi: 10.3233/bir-1976-13106. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Sugano H., Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968 Jun;59(3):251–258. [PubMed] [Google Scholar]
- Payne J. H., DeWind L. T. Surgical treatment of obesity. Am J Surg. 1969 Aug;118(2):141–147. doi: 10.1016/0002-9610(69)90113-5. [DOI] [PubMed] [Google Scholar]
- Prior P., Gyde S. N., Macartney J. C., Thompson H., Waterhouse J. A., Allan R. N. Cancer morbidity in ulcerative colitis. Gut. 1982 Jun;23(6):490–497. doi: 10.1136/gut.23.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Smith B., Butler M. The autonomic control of colonic mucin secretion in the mouse. Br J Exp Pathol. 1974 Dec;55(6):615–621. [PMC free article] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Proximal enterectomy stimulates distal hyperplasia more than bypass or pancreaticobiliary diversion. Gastroenterology. 1978 Jan;74(1):16–23. [PubMed] [Google Scholar]
- Williamson R. C., Davies P. W., Bristol J. B., Wells M. Intestinal adaptation and experimental carcinogenesis after partial colectomy. Increased tumour yields are confined to the anastomosis. Gut. 1982 Apr;23(4):316–325. doi: 10.1136/gut.23.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]



