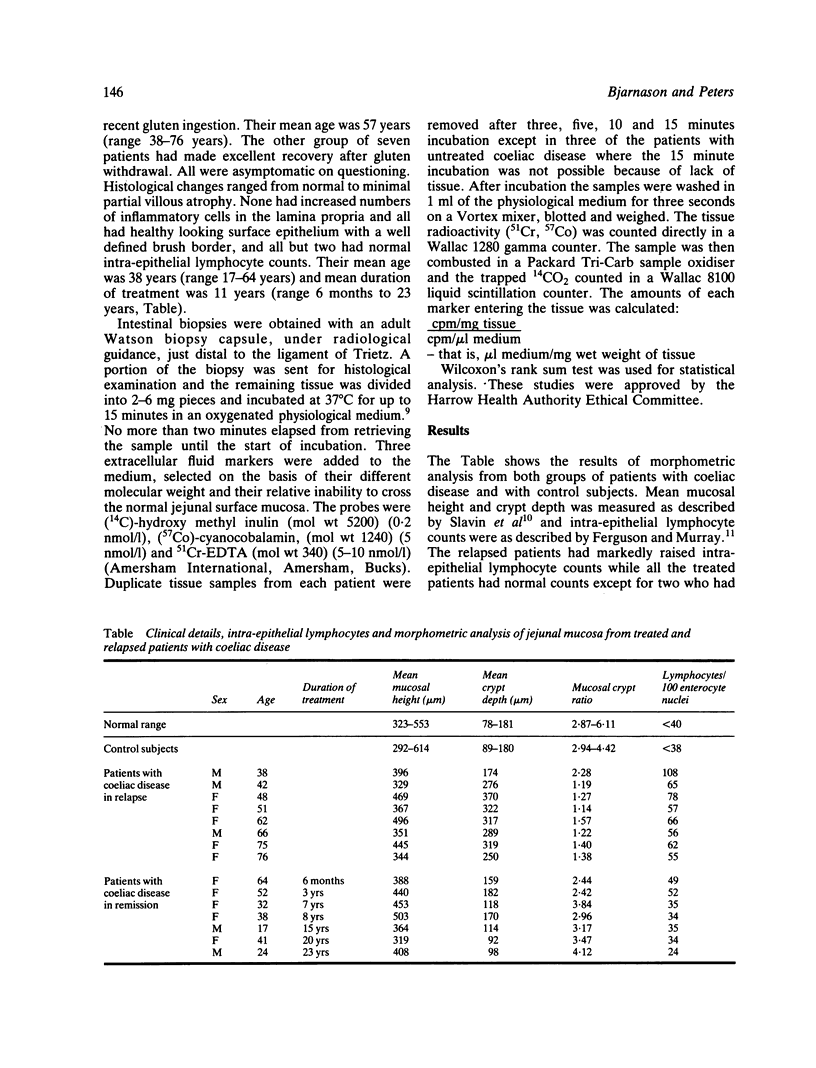
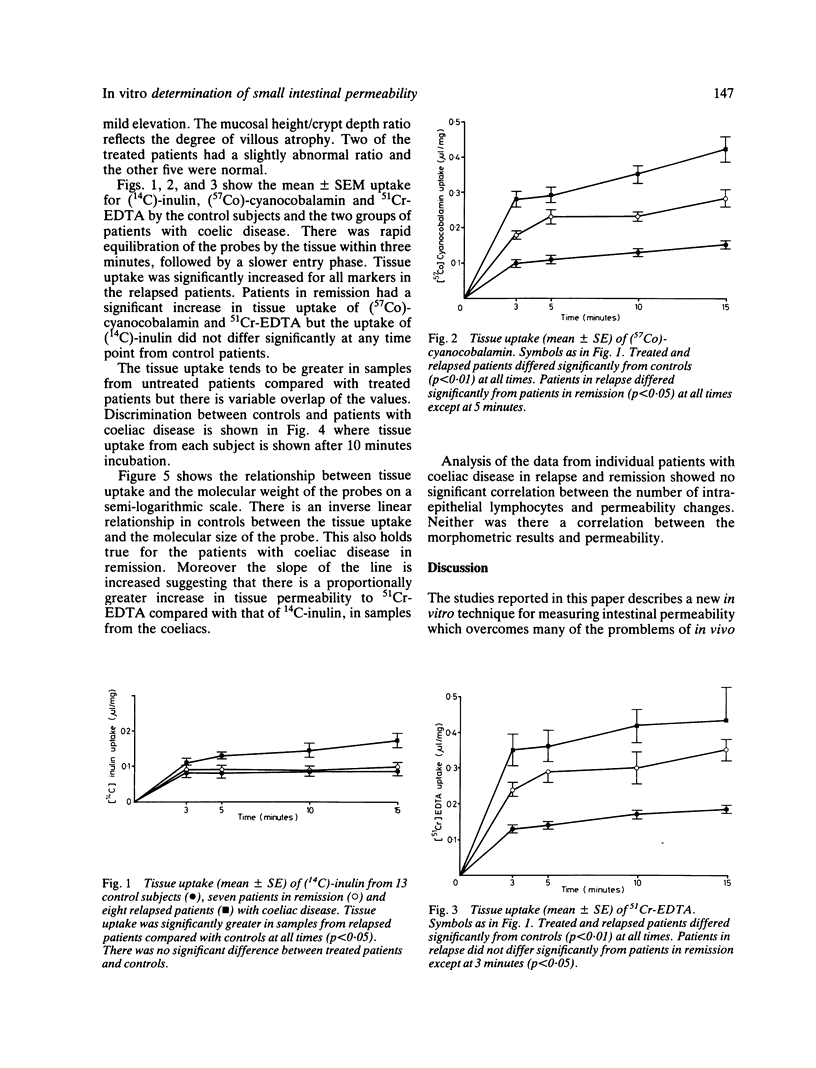
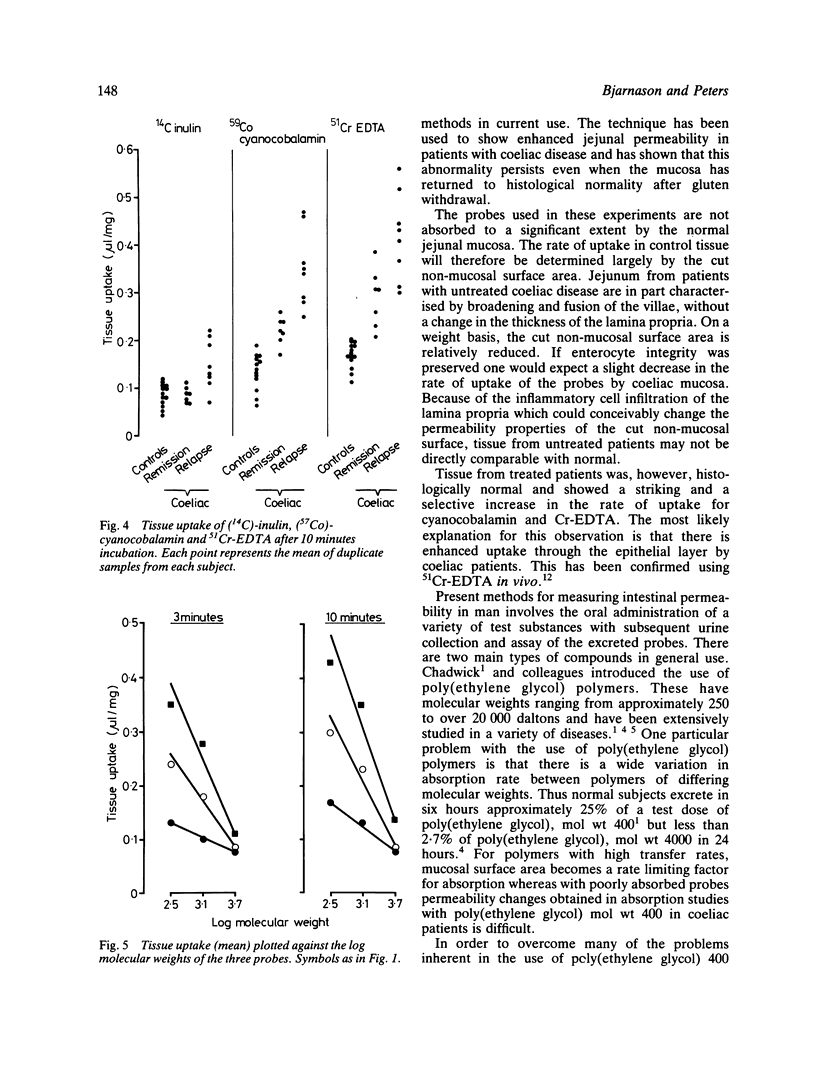
Abstract
Previous methods for measuring intestinal permeability involve the urinary measurements of various poorly digested sugar or inert poly(ethylene glycol) polymers after their oral administration. The results reflect a variety of gastrointestinal parameters including transit time, mucosal surface area and transfer, mesenteric blood and/or lymphatic flow and renal function, as well as mucosal permeability. A new in vitro method for direct measurements of mucosal permeability to three probes is described and permeability is shown to be inversely related to the molecular weight of the probe molecule. Using this technique, a persistent increase in mucosal permeability to certain probes (molecular weight less than 1500 daltons) has been shown in patients with coeliac disease in whom treatment by strict gluten withdrawal has been accompanied by restoration of intestinal histological normality. It is suggested that this finding represents a primary defect in this syndrome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., Peters T. J., Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983 Feb 12;1(8320):323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- Bronstein H. D., Haeffner L. J., Kowlessar O. D. Enzymatic digestion of gliadin: the effect of the resultant peptides in adult celiac disease. Clin Chim Acta. 1966 Aug;14(2):141–155. doi: 10.1016/0009-8981(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology. 1977 Aug;73(2):247–251. [PubMed] [Google Scholar]
- Cobden I., Dickinson R. J., Rothwell J., Axon A. T. Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. Br Med J. 1978 Oct 14;2(6144):1060–1060. doi: 10.1136/bmj.2.6144.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Peters T. J. The kinetics of iron uptake in vitro by human duodenal mucosa: studies in normal subjects. J Physiol. 1979 Apr;289:469–478. doi: 10.1113/jphysiol.1979.sp012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I., Cobden I., Rothwell J., Axon A. T. Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut. 1982 Mar;23(3):202–210. doi: 10.1136/gut.23.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. G., Lessof M. H., Baker R. W., Ferrett J., MacDonald D. M. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981 Jun 13;1(8233):1285–1286. doi: 10.1016/s0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. The small intestine: mechanisms of local immunity and gluten sensitivity. Clin Sci (Lond) 1981 Nov;61(5):497–503. doi: 10.1042/cs0610497. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Investigation of tissue organelles by a combination of analytical subcellular fractionation and enzymic microanalysis: a new approach to pathology. J Clin Pathol. 1981 Jan;34(1):1–12. doi: 10.1136/jcp.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Jones P. E., Wells G. Analytical subcellular fractionation of jejunal biopsy specimens: enzyme activities, organelle pathology and response to gluten withdrawal in patients with coeliac disease. Clin Sci Mol Med. 1978 Sep;55(3):285–292. doi: 10.1042/cs0550285. [DOI] [PubMed] [Google Scholar]
- Robinson G. M., Orrego H., Israel Y., Devenyi P., Kapur B. M. Low-molecular-weight polyethylene glycol as a probe of gastrointestinal permeability after alcohol ingestion. Dig Dis Sci. 1981 Nov;26(11):971–977. doi: 10.1007/BF01314757. [DOI] [PubMed] [Google Scholar]
- Schmid W. C., Phillips S. F., Summerskill W. H. Jejunal secretion of electrolytes and water in nontropical sprue. J Lab Clin Med. 1969 May;73(5):772–783. [PubMed] [Google Scholar]
- Slavin G., Sowter C., Robertson K., McDermott S., Paton K. Measurement in jejunal biopsies by computer-aided microscopy. J Clin Pathol. 1980 Mar;33(3):254–261. doi: 10.1136/jcp.33.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]


