Abstract
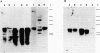
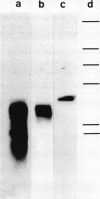
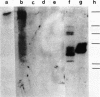
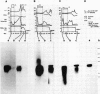
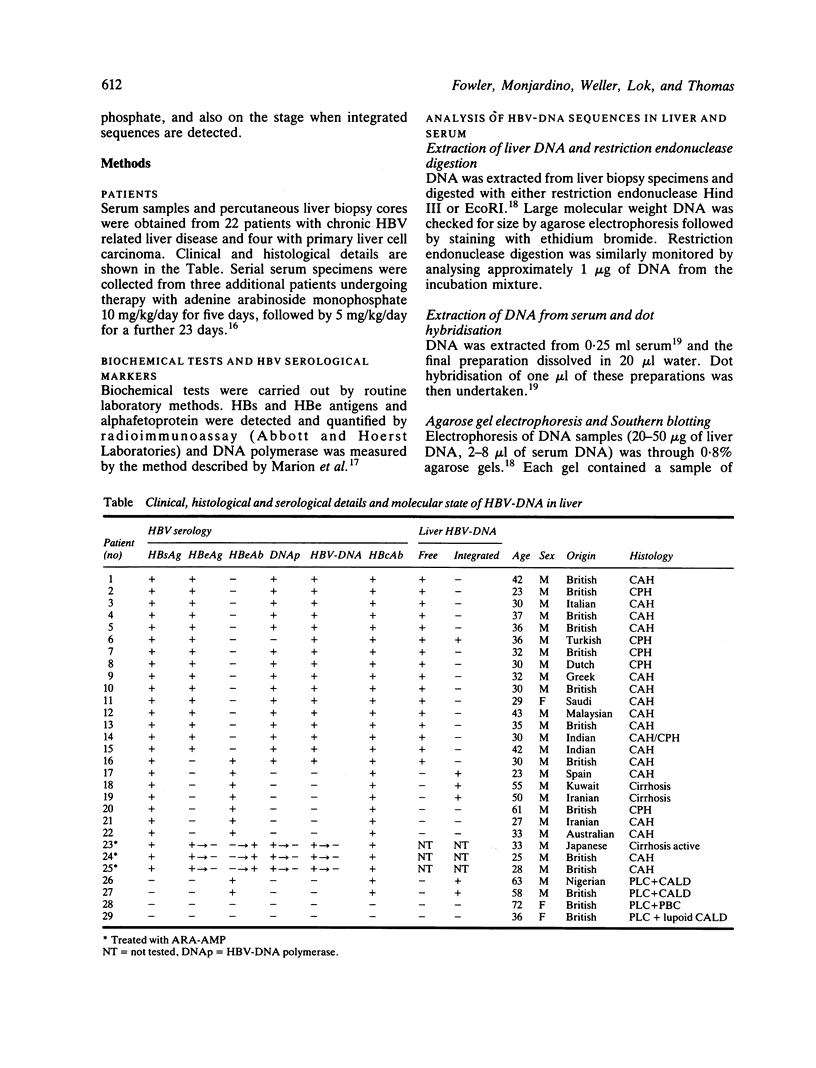
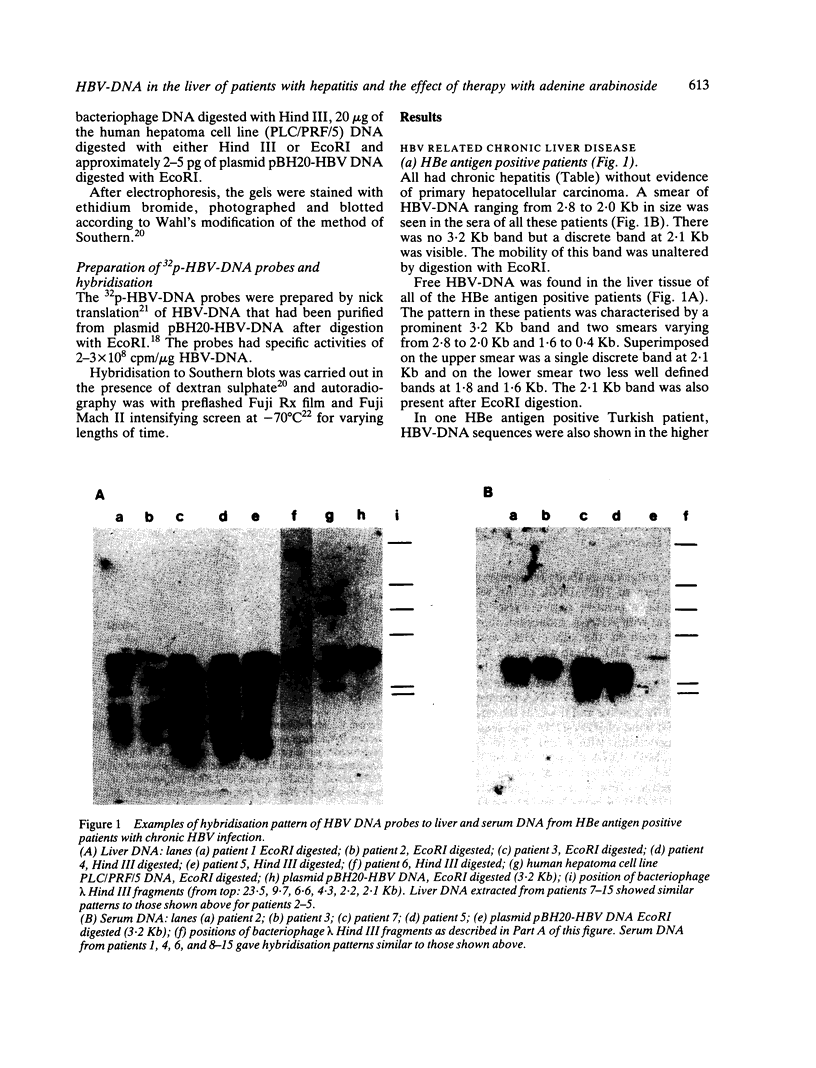
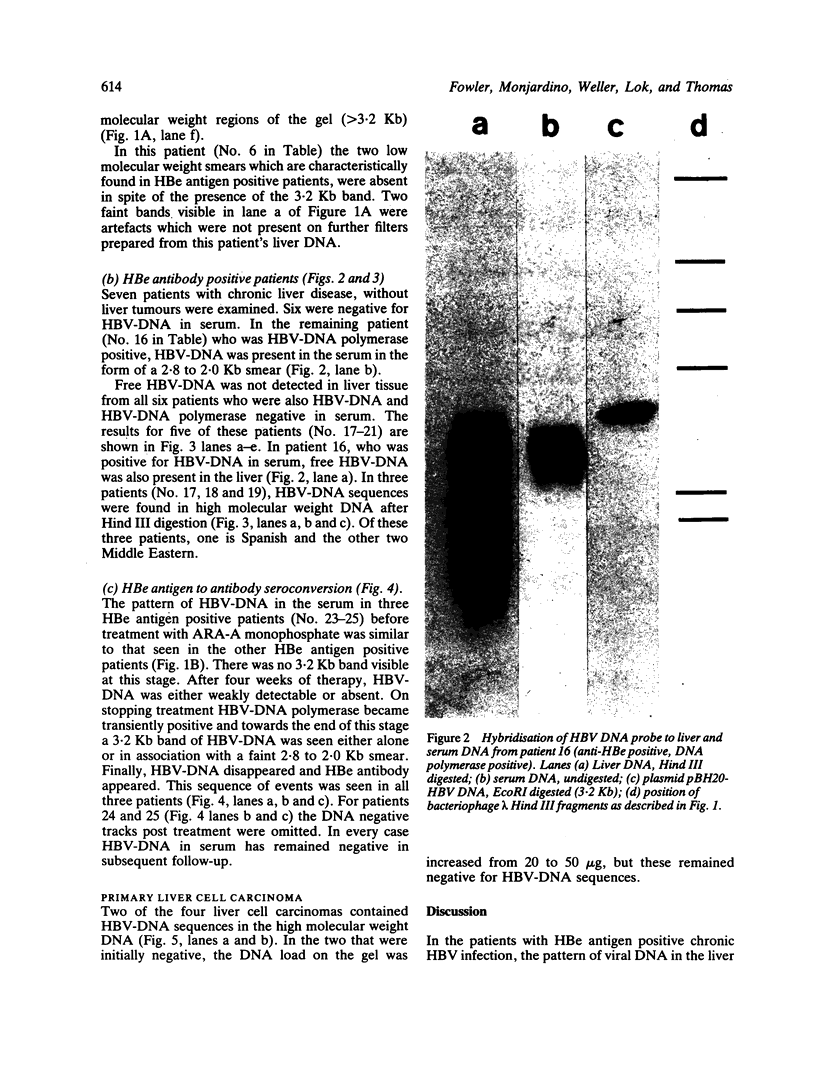
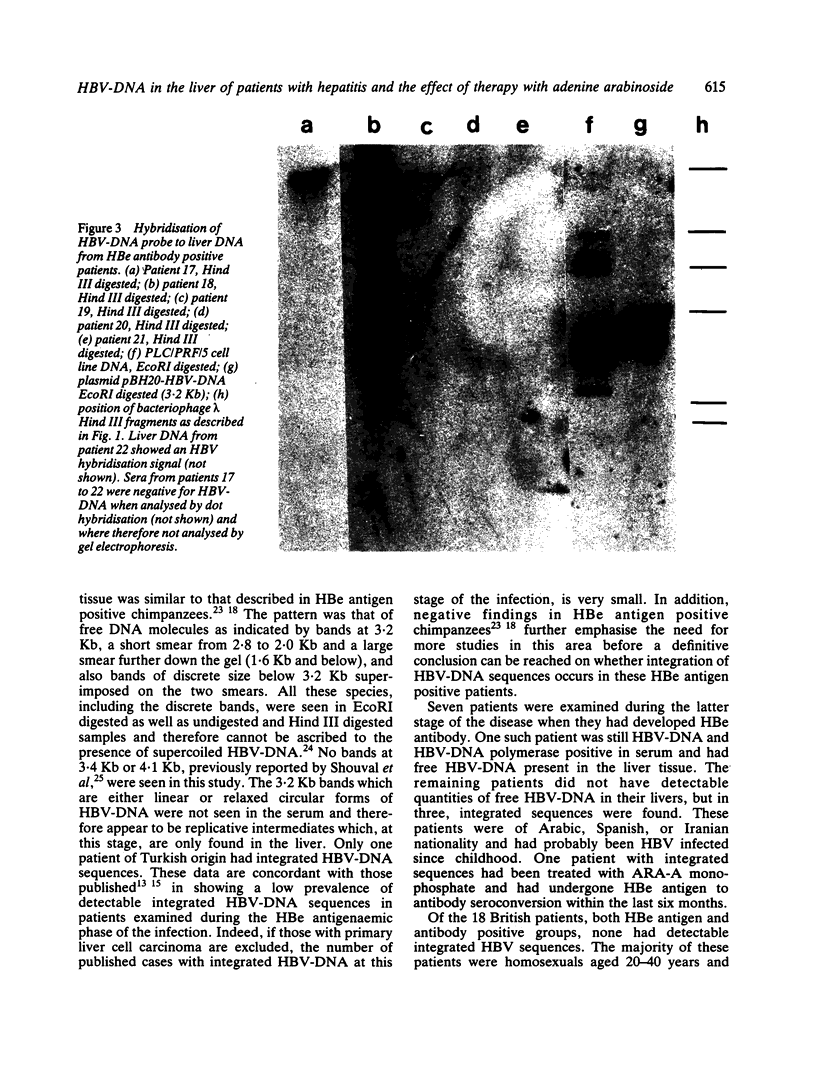
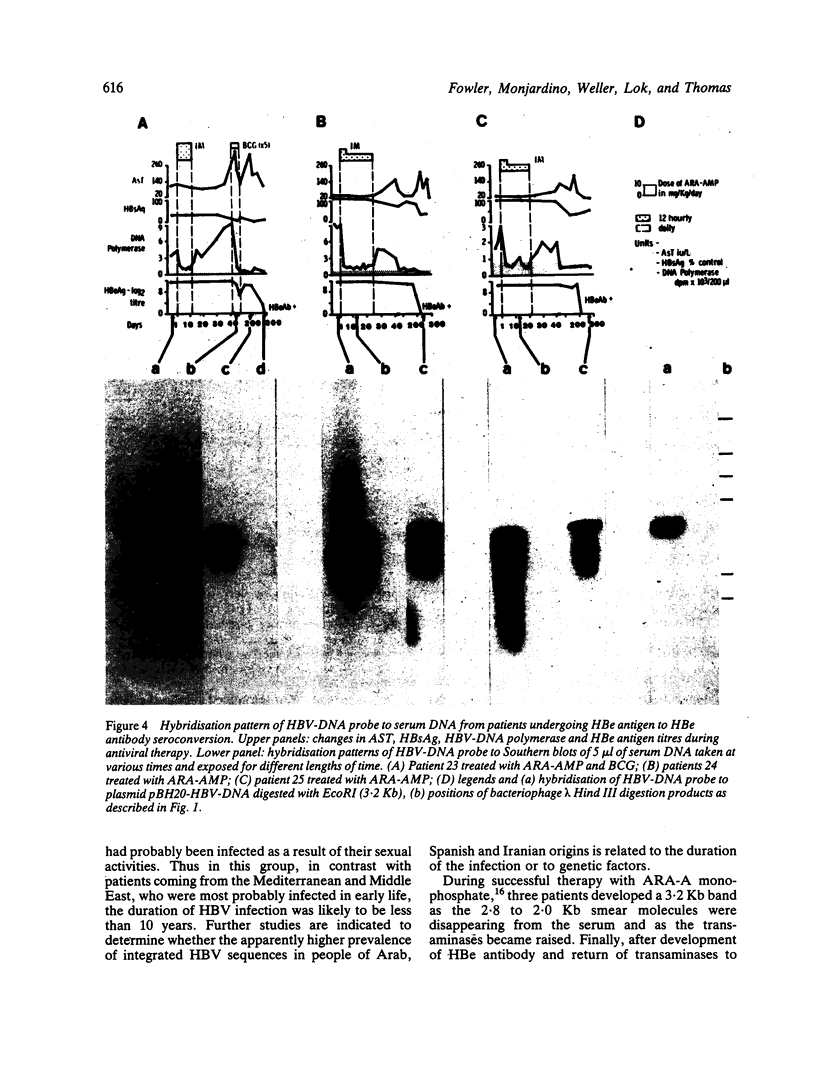
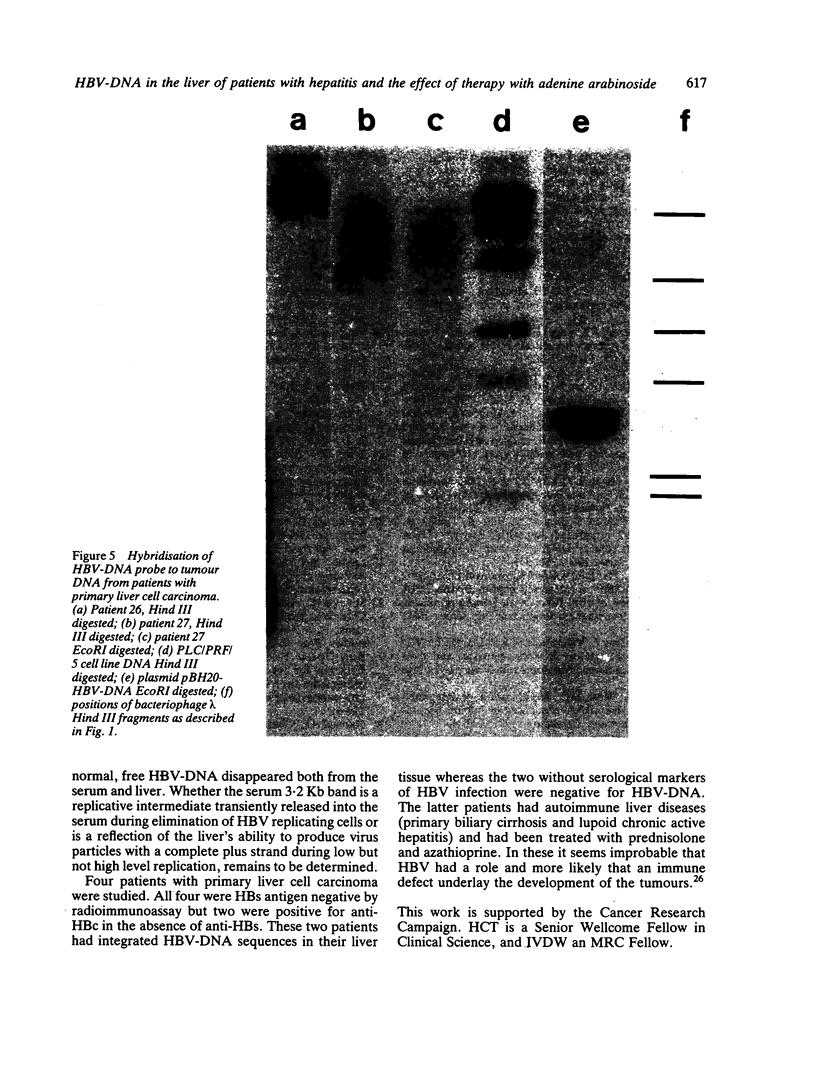
The pattern of replicative intermediates seen in the liver of HBe antigen and antibody positive patients was determined. During the phase of HBe antigenaemia the 3.2 Kb species of HBV-DNA (complete HBV genome) is present in the liver but not in the serum. When HBe antigen to antibody seroconversion occurs, either spontaneously or during antiviral therapy, the 3.2 Kb and lower molecular weight intermediates disappear from the liver and the 3.2 Kb band appears transiently in the serum. Integrated HBV-DNA was found in one of 15 patients during the period of HBe antigenaemia and in three of seven patients in the HBe antibody positive phase of the chronic infection before detection of primary liver cell carcinoma. Integrated sequences were found in tumour tissue of two patients with primary liver cell carcinoma who were anti-HBc positive but were absent from the tissues of two patients developing primary liver cell carcinoma at a late stage of autoimmune liver disease. These studies suggest that integration of the HBV genome occurs rarely or in only a small proportion of hepatocytes during the early (HBe antigen positive) phase of infection in Caucasians. They also show that not all primary liver cell carcinomas necessarily contain HBV-DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassendine M. F., Chadwick R. G., Lyssiotis T., Thomas H. C., Sherlock S., Cohen B. J. Primary liver cell cancer in Britain--a viral aetiology? Br Med J. 1979 Jan 20;1(6157):166–166. doi: 10.1136/bmj.1.6157.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassendine M. F., Della Seta L., Salmeron J., Thomas H. C., Sherlock S. Incidence of hepatitis B virus infection in alcoholic liver disease, HBsAg negative chronic active liver disease and primary liver cell cancer in Britain. Liver. 1983 Apr;3(2):65–70. doi: 10.1111/j.1600-0676.1983.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Lin C. C., Chien C. S., Chen C. J., Hwang L. Y. Geographic distribution of HBsAg carriers in China. Hepatology. 1982 Sep-Oct;2(5):553–556. doi: 10.1002/hep.1840020507. [DOI] [PubMed] [Google Scholar]
- Brechot C., Hadchouel M., Scotto J., Degos F., Charnay P., Trepo C., Tiollais P. Detection of hepatitis B virus DNA in liver and serum: a direct appraisal of the chronic carrier state. Lancet. 1981 Oct 10;2(8250):765–768. doi: 10.1016/s0140-6736(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Bréchot C., Hadchouel M., Scotto J., Fonck M., Potet F., Vyas G. N., Tiollais P. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3906–3910. doi: 10.1073/pnas.78.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs A. K., Bassendine M. F., Thomas H. C., Sherlock S. Primary liver cell cancer in autoimmune chronic liver disease. Br Med J (Clin Res Ed) 1981 Jan 24;282(6260):273–273. doi: 10.1136/bmj.282.6260.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. R., Ruiz-Opazo N., Shouval D., Shafritz D. A. Identification of integrated hepatitis B virus DNA and expression of viral RNA in an HBsAg-producing human hepatocellular carcinoma cell line. Nature. 1980 Jul 31;286(5772):531–533. doi: 10.1038/286531a0. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjardino J., Fowler M. J., Montano L., Weller I., Tsiquaye K. N., Zuckerman A. J., Jones D. M., Thomas H. C. Analysis of hepatitis virus DNA in the liver and serum of HBe antigen positive chimpanzee carriers. J Med Virol. 1982;9(3):189–199. doi: 10.1002/jmv.1890090306. [DOI] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Chakraborty P. R., Shafritz D. A. Evidence for supercoiled hepatitis B virus DNA in chimpanzee liver and serum Dane particles: possible implications in persistent HBV infection. Cell. 1982 May;29(1):129–136. doi: 10.1016/0092-8674(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Kew M. C. Identification of integrated hepatitis B virus DNA sequences in human hepatocellular carcinomas. Hepatology. 1981 Jan-Feb;1(1):1–8. doi: 10.1002/hep.1840010102. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Shouval D., Sherman H. I., Hadziyannis S. J., Kew M. C. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981 Oct 29;305(18):1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- Shouval D., Chakraborty P. R., Ruiz-Opazo N., Baum S., Spigland I., Muchmore E., Gerber M. A., Thung S. N., Popper H., Shafritz D. A. Chronic hepatitis in chimpanzee carriers of hepatitis B virus: morphologic, immunologic, and viral DNA studies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6147–6151. doi: 10.1073/pnas.77.10.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval D., Chakraborty P. R., Ruiz-Opazo N., Baum S., Spigland I., Muchmore E., Gerber M. A., Thung S. N., Popper H., Shafritz D. A. Chronic hepatitis in chimpanzee carriers of hepatitis B virus: morphologic, immunologic, and viral DNA studies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6147–6151. doi: 10.1073/pnas.77.10.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tabor E., Gerety R. J., Vogel C. L., Bayley A. C., Anthony P. P., Chan C. H., Barker L. F. Hepatitis B virus infection and primary hepatocellular carcinoma. J Natl Cancer Inst. 1977 May;58(5):1197–1200. doi: 10.1093/jnci/58.5.1197. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller I. V., Bassendine M. F., Craxi A., Fowler M. J., Monjardino J., Thomas H. C., Sherlock S. Successful treatment of HBs and HBeAg positive chronic liver disease: prolonged inhibition of viral replication by highly soluble adenine arabinoside 5'-monophosphate (ARA-AMP). Gut. 1982 Sep;23(9):717–723. doi: 10.1136/gut.23.9.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller I. V., Fowler M. J., Monjardino J., Thomas H. C. The detection of HBV-DNA in serum by molecular hybridisation: a more sensitive method for the detection of complete HBV particles. J Med Virol. 1982;9(4):273–280. doi: 10.1002/jmv.1890090405. [DOI] [PubMed] [Google Scholar]