Abstract
The concentration of IgG and IgA was measured in the supernatants of peripheral blood mononuclear cells and of cells harvested from the intestinal lamina propria, which were cultured in vitro in the presence or absence of mitogens. The lamina propria mononuclear cells were harvested by collagenase digestion of macroscopically normal mucosa from 10 fresh surgical resections for carcinoma. Secretion of IgA in cultures of unstimulated lamina propria mononuclear cells greatly exceeded that of IgG. The addition of pokeweed mitogen increased Ig secretion by cultures of peripheral blood mononuclear cells but decreased Ig secretion by lamina propria mononuclear cells. The addition of concanavalin A suppressed Ig synthesis by pokeweed mitogen stimulated cells more in cultures of peripheral blood mononuclear cells than in lamina propria mononuclear cells. Cycloheximide inhibited Ig secretion by more than 90% in cultures of peripheral blood mononuclear cells, but there was less inhibition in cultures of lamina propria mononuclear cells. In the four unstimulated cultures of lamina propria mononuclear cells examined, over 75% of the Ig was secreted in the first three to four days of culture. The results indicate that lamina propria mononuclear cells are refractory to the inductive and suppressive signals of mitogens, and represent an activated cell population which is committed to Ig secretion before being cultured.
Full text
PDF

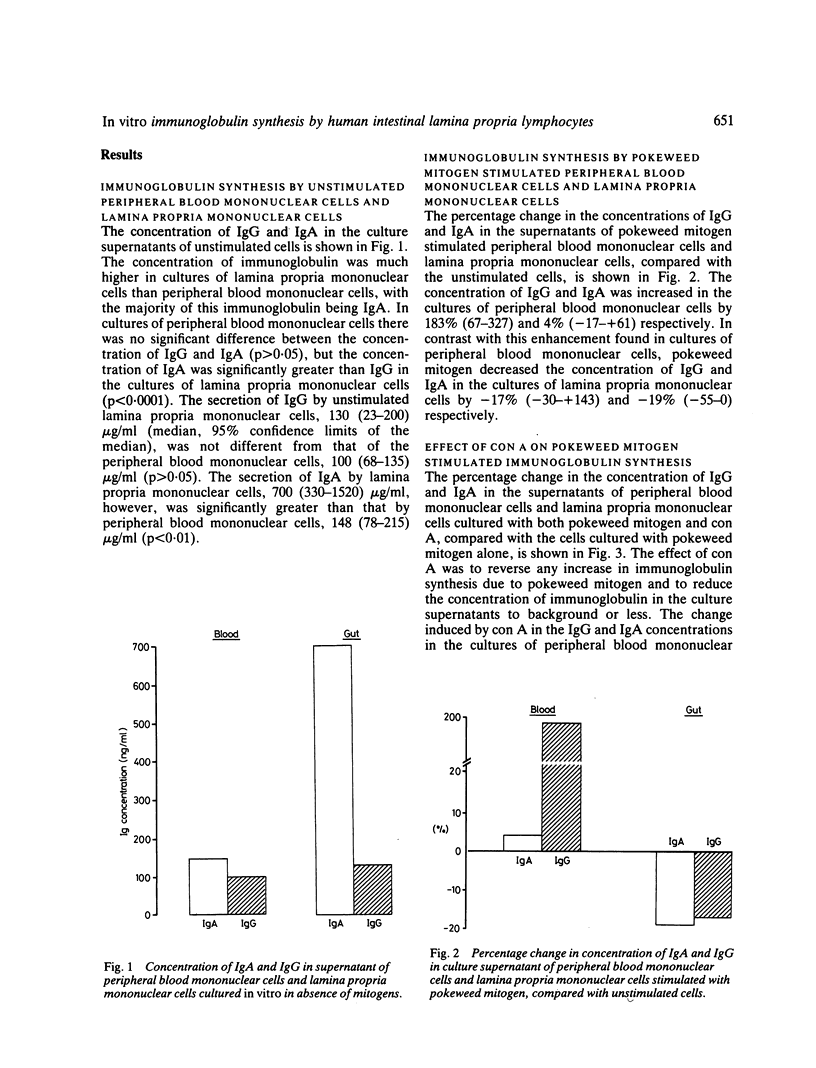
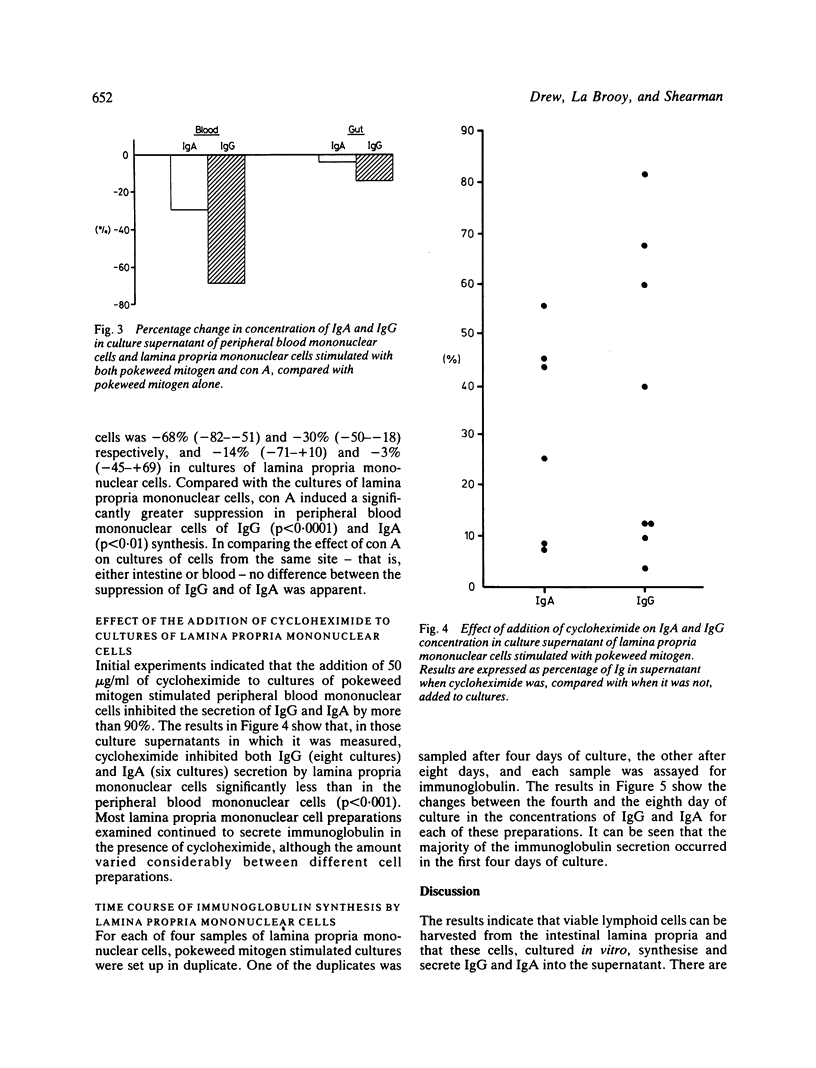
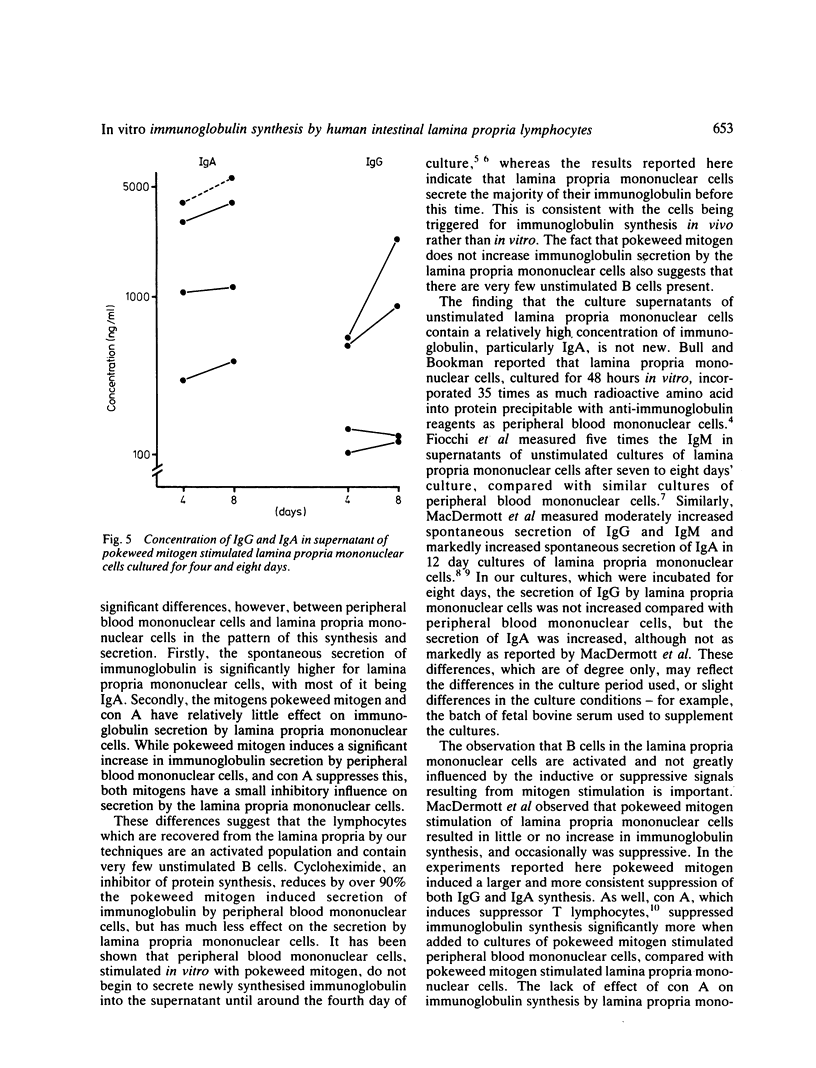


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister K. M., Drew P. A., Clarkson A. R., Woodroffe A. J. Immunoregulation in glomerulonephritis, Henoch--Schonlein purpura and lupus nephritis. Clin Exp Immunol. 1983 Aug;53(2):384–390. [PMC free article] [PubMed] [Google Scholar]
- Beale M. G., Nash G. S., Bertovich M. J., MacDermott R. P. Similar disturbances in B cell activity and regulatory T cell function in Henoch-Schönlein purpura and systemic lupus erythematosus. J Immunol. 1982 Jan;128(1):486–491. [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Choi Y. S., Good R. A. Differentiation of human peripheral blood B lymphocytes. Immunology. 1977 Dec;33(6):887–894. [PMC free article] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Suppressor T cells. Transplant Rev. 1975;26:39–55. doi: 10.1111/j.1600-065x.1975.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Heck J. A., Strober W. T-cell regulation of murine IgA synthesis. J Exp Med. 1979 Mar 1;149(3):632–643. doi: 10.1084/jem.149.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C., Battisto J. R., Farmer R. G. Gut mucosal lymphocytes in inflammatory bowel disease: isolation and preliminary functional characterization. Dig Dis Sci. 1979 Sep;24(9):705–717. doi: 10.1007/BF01314469. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Keren D. F., Yardley J. H., Brown G. No impairment of local intestinal immune response to keyhole limpet haemocyanin in the absence of Peyer's patches. Immunology. 1981 Mar;42(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Heddle R. J., La Brooy J. T., Shearman D. J. Escherichia coli antibody-secreting cells in the human intestine. Clin Exp Immunol. 1982 May;48(2):469–476. [PMC free article] [PubMed] [Google Scholar]
- Holdstock G., Ershler W. B., Krawitt E. L. Demonstration of non-specific B-cell stimulation in patients with cirrhosis. Gut. 1982 Sep;23(9):724–728. doi: 10.1136/gut.23.9.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Munoz J., Pryjma J., Fudenberg H. H., Virella G. Immunoglobulin quantitation and enumeration of immunoglobulin-producing cells: comparison of two index of B-cell activation. Scand J Immunol. 1980;12(4):345–353. doi: 10.1111/j.1365-3083.1980.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]


