Abstract
Perhexiline maleate is an antianginal agent which depends on hepatic oxidation for its elimination. Its use may be complicated by the development of peripheral neuropathy and liver damage. The majority of patients with perhexiline neuropathy have an impaired ability to effect metabolic drug oxidation which is genetically determined. Information has not been available on drug oxidation capacity in patients with perhexiline liver injury. Drug oxidation was measured using an oxidation phenotyping procedure in four patients with perhexiline liver injury and in 70 patients with chronic liver disease serving as a control group. All four patients with perhexiline liver damage showed a substantial metabolic defect; three of the four patients (75%) showed a genetically determined impairment of oxidation capacity. The incidence of severely impaired oxidation capacity in the perhexiline group was significantly greater than in the patients with chronic liver disease (6/70; 8.6%) and in the healthy population (9%) (F = 0.0048). A clear association exists between perhexiline liver injury and diminished drug metabolic activity, suggesting that the propensity to develop perhexiline liver injury is, at least in part, genetically determined.
Full text
PDF

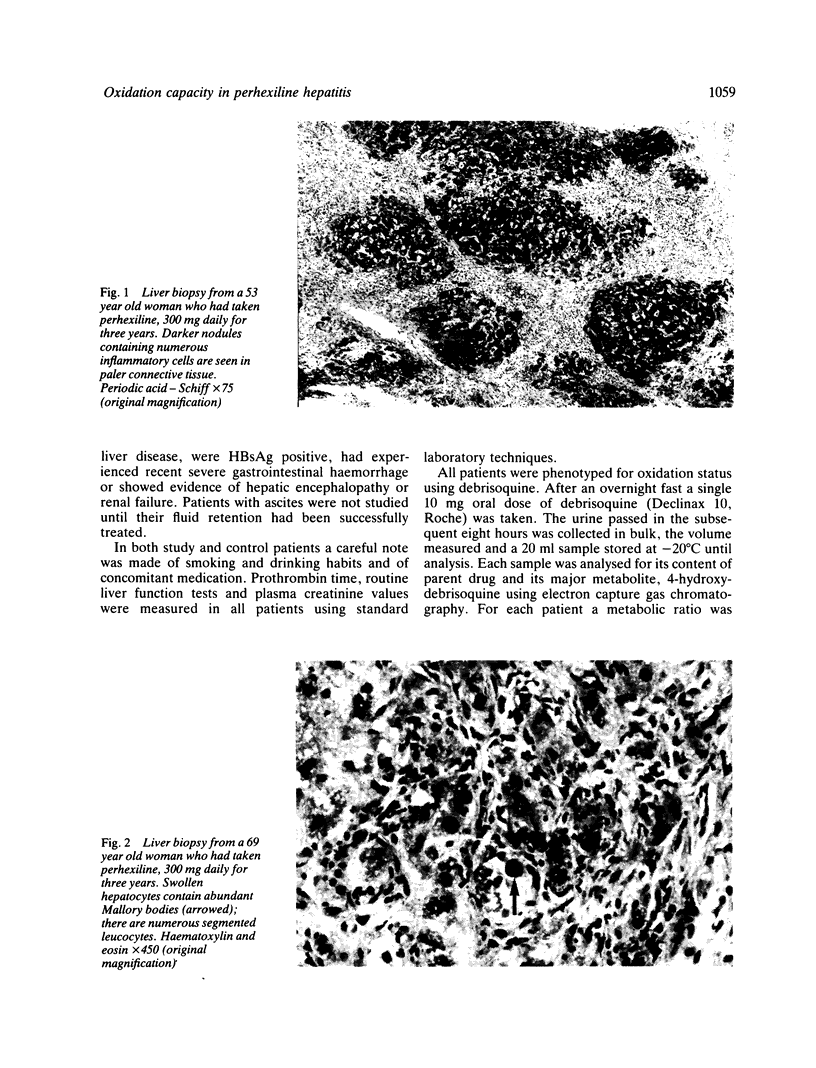
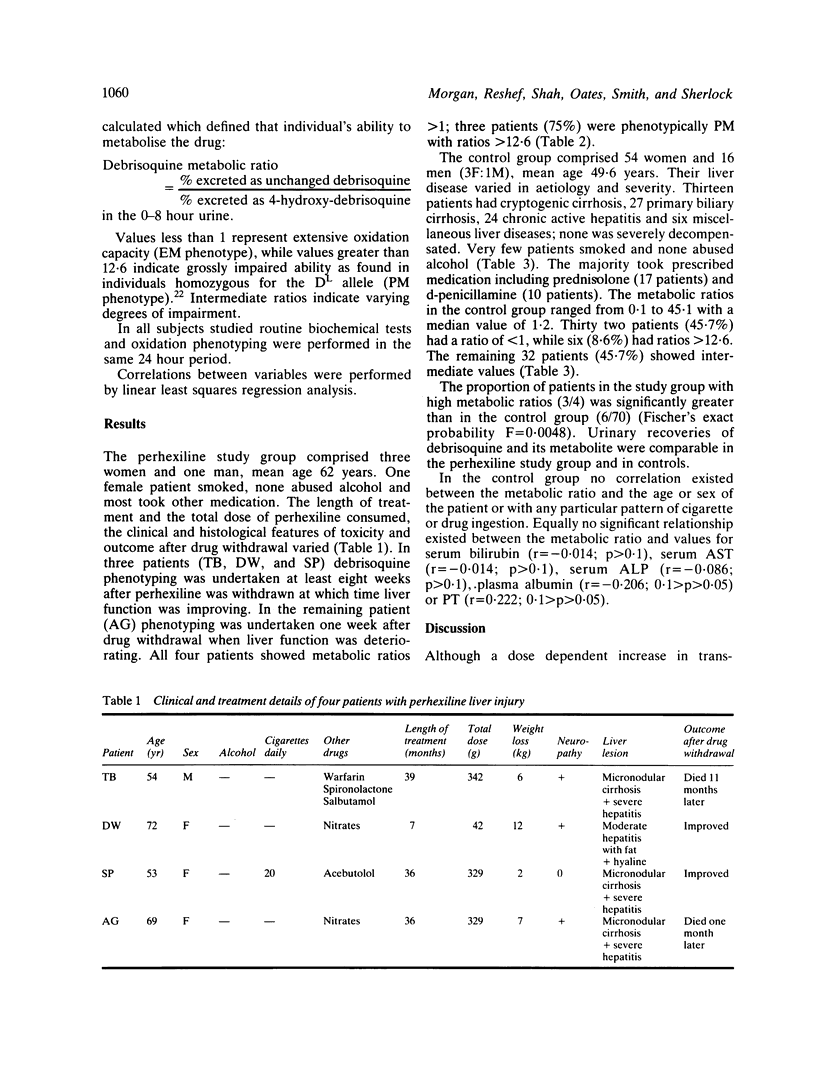
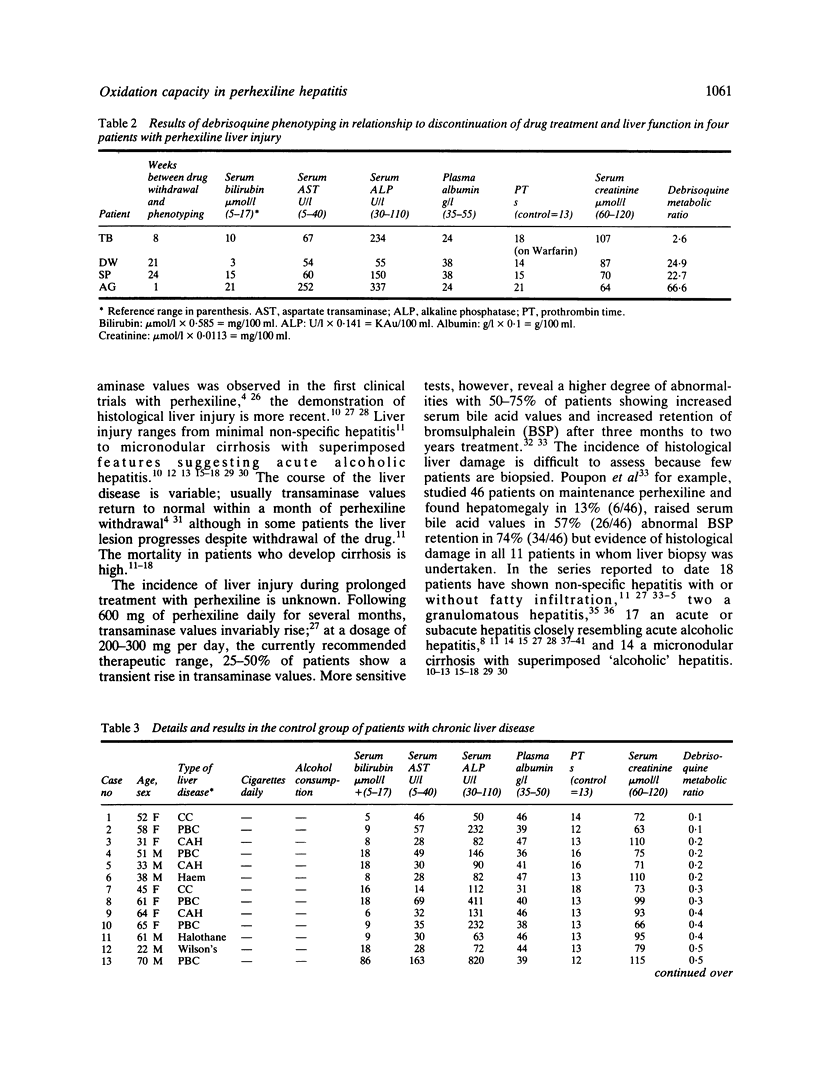
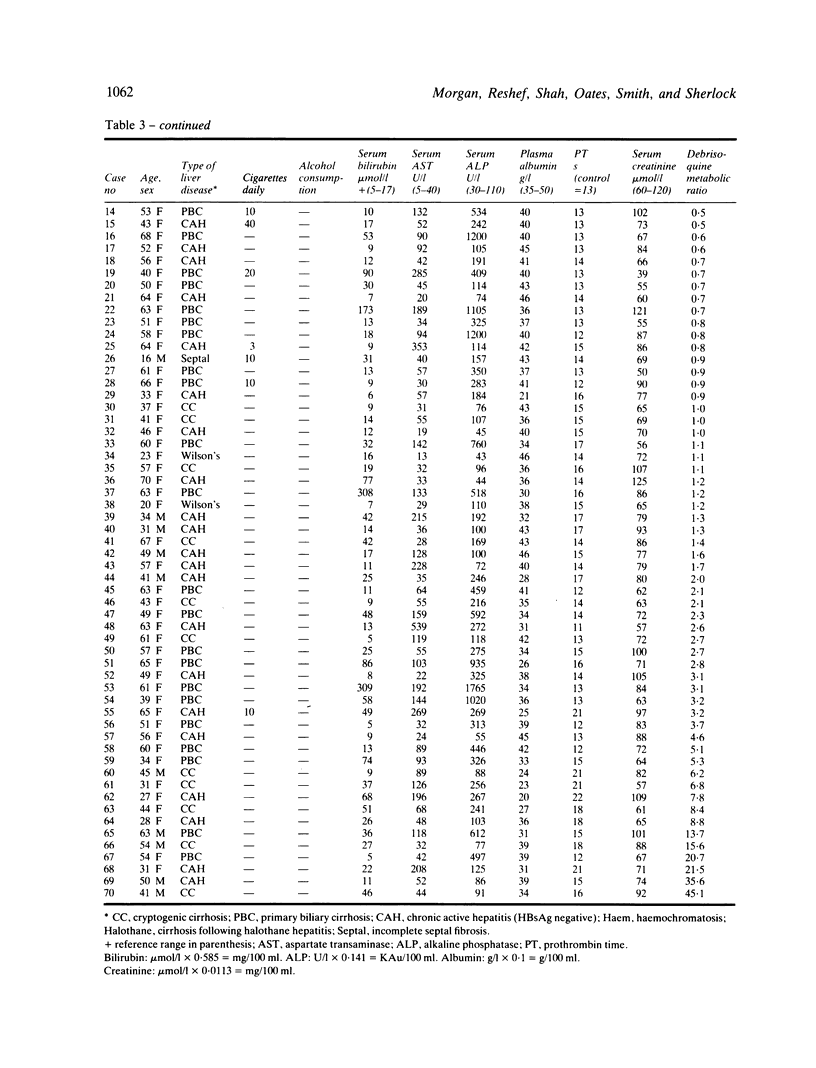


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abaza A., Cattan D., Aziza C., Pappo E. Lettre: Effets secondaires mais réversibles á la prise de perhexiline. Nouv Presse Med. 1973 Nov 24;2(42):2820–2820. [PubMed] [Google Scholar]
- Beaugrand M., Chousterman M., Callard P., Camilleri J. P., Petite J., Ferrier J. P. Hépatites au maléate de perhexiline (Pexid). Evoluant vers la cirrhose malgré l'arrêt du traitement (2 cas). Gastroenterol Clin Biol. 1977 Oct;1(10):745–750. [PubMed] [Google Scholar]
- Beaugrand M. Hépatite médicamenteuse à la perhexilline. Nouv Presse Med. 1976 Jul 3;5(27):1695–1695. [PubMed] [Google Scholar]
- Beaugrand M., Poupon R., Lévy V. G., Callard P., Lageron A., Lecomte D., Darnis F., Ferrier J. P. Lésions hépatiques dues au maléate de perhexiline. Etude clinique, biologique, histopathologique, ultrastructurale et biochimique de 7 cas. Gastroenterol Clin Biol. 1978;2(6-7):579–588. [PubMed] [Google Scholar]
- Blanchon P., Paillas J., Lenoir C., Vieillefond A., Lauriat H. Documents anatomiques concernant un accident mortel dû à un traitement prolongé par le maléate de perhexiline. Ann Med Interne (Paris) 1977 Aug-Sep;128(8-9):715–718. [PubMed] [Google Scholar]
- Bonnet P., Jourdan J., Janbon F., Michel H., Bertrand A. Cirrhose hépatique après deux ans de traitement par le maléate de perhexiline. Nouv Presse Med. 1978 Jan 21;7(3):208–208. [PubMed] [Google Scholar]
- Dawes P., Moulder C. Perhexiline hepatitis and HLA-B8. Lancet. 1982 Jul 10;2(8289):109–109. doi: 10.1016/s0140-6736(82)91736-6. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G. B., Rake M. O., Taylor D. J. Liver damage due to perhexiline maleate. J Clin Pathol. 1979 Dec;32(12):1282–1285. doi: 10.1136/jcp.32.12.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw J. J., Singer B., Poupon R., Lecomte D., de Saint-Maur P. P., Lageron A., Pollet S. Inclusions polymorphes diffuses chez un malade traité par le maléate de perhexiline. Nouv Presse Med. 1978 Mar 11;7(10):817–820. [PubMed] [Google Scholar]
- Herne N., Raillat A., Marion J., Le Gall F., Chagnon A. Hépatite toxique sub-aiguë et maléate de perhexilline. Nouv Presse Med. 1976 Oct 9;5(9 OCT):2164–2165. [PubMed] [Google Scholar]
- Houdent C. E., Wolf L. M., Corriat A. Liver during perhexiline hypoglycaemia. Lancet. 1977 Nov 12;2(8046):1028–1028. doi: 10.1016/s0140-6736(77)92922-1. [DOI] [PubMed] [Google Scholar]
- Kahn G. C., Boobis A. R., Murray S., Brodie M. J., Davies D. S. Assay and characterisation of debrisoquine 4-hydroxylase activity of microsomal fractions of human liver. Br J Clin Pharmacol. 1982 May;13(5):637–645. doi: 10.1111/j.1365-2125.1982.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman P., Morgan P. G. Liver damage after perhexiline maleate. Lancet. 1977 Mar 26;1(8013):705–705. doi: 10.1016/s0140-6736(77)92149-3. [DOI] [PubMed] [Google Scholar]
- Lenoir C., Blanchon P. Hépatites dues au maléate de perhexiline. Coeur Med Interne. 1978 Jan-Mar;17(1):69–75. [PubMed] [Google Scholar]
- Lewis D., Wainwright H. C., Kew M. C., Zwi S., Isaacson C. Liver damage associated with perhexiline maleate. Gut. 1979 Mar;20(3):186–189. doi: 10.1136/gut.20.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther. 1972 May;181(2):279–287. [PubMed] [Google Scholar]
- Long-term perhexiline maleate and liver function. Br Med J. 1976 Jan 17;1(6002):133–133. doi: 10.1136/bmj.1.6002.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. P., Fitzgerald O., Maurer B. J. Hepatotoxicity following treatment with perhexiline maleate. Ir Med J. 1980 Jul;73(7):275–276. [PubMed] [Google Scholar]
- Lubetzki J., Warnet A., Kaloustian E., Fusciardi J. Complications multiples d'un traitement par le maléate de perhexiline. Ann Med Interne (Paris) 1977 Aug-Sep;128(8-9):719–722. [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Paliard P., Vitrey D., Fournier G., Belhadjali J., Patricot L., Berger F. Perhexiline maleate-induced hepatitis. Digestion. 1978;17(5):419–427. doi: 10.1159/000198145. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Lambert R., Paliard P., Bory R. Hépatite toxique subaiguë après traitement par maléate de perhexiline. Nouv Presse Med. 1976 Apr 17;5(16):1070–1070. [PubMed] [Google Scholar]
- Pessayre D., Bichara M., Degott C., Potet F., Benhamou J. P., Feldmann G. Perhexiline maleate-induced cirrhosis. Gastroenterology. 1979 Jan;76(1):170–177. [PubMed] [Google Scholar]
- Poupon R., Rosensztajn L., Prudhomme de Saint-Maur P., Lageron A., Gombeau T., Darnis F. Perhexiline maleate-associated hepatic injury prevalence and characteristics. Digestion. 1980;20(3):145–150. doi: 10.1159/000198433. [DOI] [PubMed] [Google Scholar]
- Roche J., Fournet J., Meullenet J., Faure H., Massot C. Insuffisance hépatique aiguë due au maléate de perhexiline. Nouv Presse Med. 1979 Feb 10;8(7):521–522. [PubMed] [Google Scholar]
- Shah R. R., Oates N. S., Idle J. R., Smith R. L., Lockhart J. D. Impaired oxidation of debrisoquine in patients with perhexiline neuropathy. Br Med J (Clin Res Ed) 1982 Jan 30;284(6312):295–299. doi: 10.1136/bmj.284.6312.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan T. P., Mahgoub A., Lancaster R., Idle J. R., Smith R. L. Polymorphism of carbon oxidation of drugs and clinical implications. Br Med J. 1978 Sep 2;2(6138):655–657. doi: 10.1136/bmj.2.6138.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stentiford N. H. Perhexiline maleate toxicity: a case of severe jaundice. Br J Clin Pract. 1977 Sep;31(9):136–137. [PubMed] [Google Scholar]
- Tomlinson I. W., Rosenthal F. D. Proximal myopathy after perhexiline maleate treatment. Br Med J. 1977 May 21;1(6072):1319–1320. doi: 10.1136/bmj.1.6072.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]