Abstract
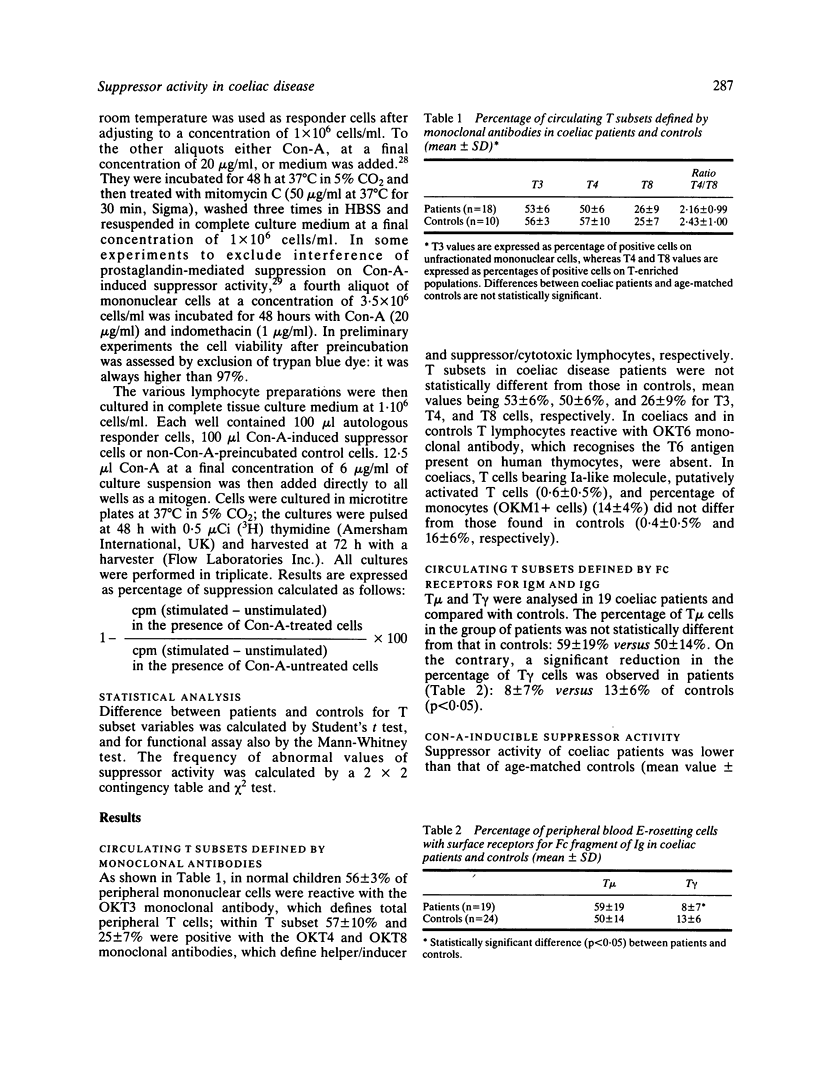
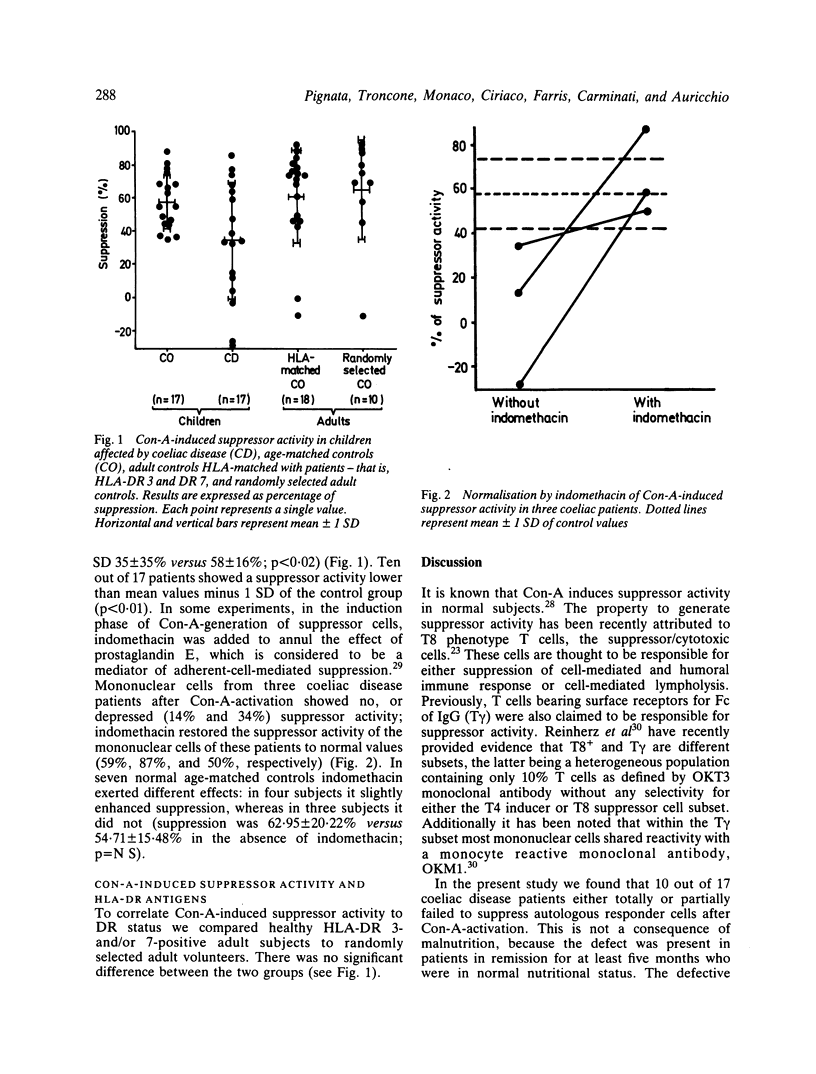
Immunoregulatory cells were enumerated in 19 coeliac disease children on a gluten free diet by means of monoclonal antibodies that define total T lymphocytes (T3), helper/inducer T cells (T4), suppressor/cytotoxic T cells (T8) and monocytes (M1), as well as by means of surface receptors for Fc fragments of IgM and IgG (T mu and T gamma, respectively). In addition, suppressor cell function was assessed in 17 coeliac disease patients by examining the ability of concanavalin-A (Con-A)-activated suppressor cells to inhibit autologous cell response to mitogenic stimulus as compared with age-matched controls. No statistically significant differences were found in the percentages of subsets defined by monoclonal antibodies between coeliac disease patients and age-matched controls, whereas coeliac disease patients had a significant decrease of the subpopulation bearing membrane receptor for Fc fragment of IgG. Mean value was 8.5% in coeliac patients versus 13.4% in age-matched controls. In the functional assay, mononuclear cells from 10 out of 17 coeliac disease patients either totally or partially failed to suppress responder cells after Con-A-activation. This defect is not related to HLA-DR status, because no difference was found between patients-HLA-matched and unmatched normal individuals. In this assay, mononuclear cells of three coeliac disease patients with low suppressor activity were able to inhibit responder cells to the same extent as controls, when indomethacin was used to block prostaglandin production in the induction phase of Con-A-activated suppressor cells. Our results suggest that an abnormality in immunoregulation may play a role in the pathogenesis of coeliac disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Idar D., Handzel Z. T., Ofarim M., Levin S. An in-vitro immunological assay for diagnosis of coeliac disease. Lancet. 1978 Mar 25;1(8065):627–629. doi: 10.1016/s0140-6736(78)91136-4. [DOI] [PubMed] [Google Scholar]
- Auricchio S., Buffolano W., Ciccimarra F., De Vincenzi M., Silano V., Zapponi G. In vitro proliferation of lymphocytes from celiac children and their first-degree relatives in response to wheat gliadin-derived peptides. J Pediatr Gastroenterol Nutr. 1982;1(4):515–524. doi: 10.1097/00005176-198212000-00011. [DOI] [PubMed] [Google Scholar]
- Betuel H., Gebuhrer L., Descos L., Percebois H., Minaire Y., Bertrand J. Adult celiac disease associated with HLA-DRw3 and -DRw7. Tissue Antigens. 1980 Mar;15(3):231–238. doi: 10.1111/j.1399-0039.1980.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Bullen A. W., Losowsky M. S. Cell-mediated immunity to gluten fraction III in adult coeliac disease. Gut. 1978 Feb;19(2):126–131. doi: 10.1136/gut.19.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi M., Borelli I., Olivetti E., Richiardi P., Wright P., Ansaldi N., Barbera C., Santini B. Two HLA-D and DR alleles are associated with coeliac disease. Tissue Antigens. 1979 Oct;14(4):309–316. doi: 10.1111/j.1399-0039.1979.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Douwes F. R. Gluten and lymphocyte sensitisation in coeliac disease. Lancet. 1976 Dec 18;2(7999):1353–1353. doi: 10.1016/s0140-6736(76)91996-6. [DOI] [PubMed] [Google Scholar]
- Falchuk Z. M., Rogentine G. N., Strober W. Predominance of histocompatibility antigen HL-A8 in patients with gluten-sensitive enteropathy. J Clin Invest. 1972 Jun;51(6):1602–1605. doi: 10.1172/JCI106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S. Modulation of conconavalin-A-induced suppressor cell activation by prostaglandin E2. Cell Immunol. 1980 Feb;49(2):421–425. doi: 10.1016/0008-8749(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Holmes G. K., Asquith P., Cooke W. T. Cell-mediated immunity to gluten fraction III in adult coeliac disease. Clin Exp Immunol. 1976 May;24(2):259–265. [PMC free article] [PubMed] [Google Scholar]
- Kenrick K. G., Walker-Smith J. A. Immunoglobulins and dietary protein antibodies in childhood coeliac disease. Gut. 1970 Aug;11(8):635–640. doi: 10.1136/gut.11.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuning J. J., Peña A. S., van Leeuwen A., van Hooff J. P., va Rood J. J. HLA-DW3 associated with coeliac disease. Lancet. 1976 Mar 6;1(7958):506–508. doi: 10.1016/s0140-6736(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Lawley T. J., Hall R. P., Fauci A. S., Katz S. I., Hamburger M. I., Frank M. M. Defective Fc-receptor functions associated with the HLA-B8/DRw3 haplotype: studies in patients with dermatitis herpetiformis and normal subjects. N Engl J Med. 1981 Jan 22;304(4):185–192. doi: 10.1056/NEJM198101223040401. [DOI] [PubMed] [Google Scholar]
- Loeb P. M., Strober W., Falchuk Z. M., Laster L. Incorporation of L-leucine-14C into immunoglobulins by jejunal biopsies of patients with celiac sprue and other gastrointestinal diseases. J Clin Invest. 1971 Mar;50(3):559–569. doi: 10.1172/JCI106525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh P., Asquith P. HLA and coeliac disease. Br Med Bull. 1978 Sep;34(3):291–294. doi: 10.1093/oxfordjournals.bmb.a071514. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata C., Vajro P., Troncone R., Monaco G., Ciriaco M. Immunoregulatory T subsets in chronic active viral hepatitis: characterization by monoclonal antibodies. J Pediatr Gastroenterol Nutr. 1983 May;2(2):229–233. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora K., Anand B. S., Truelove S. C., Ciclitira P. J., Offord R. E. Stimulation of lymphocytes from patients with coeliac disease by a subfraction of gluten. Lancet. 1976 Aug 21;2(7982):389–391. doi: 10.1016/s0140-6736(76)92406-5. [DOI] [PubMed] [Google Scholar]
- Tosi R., Vismara D., Tanigaki N., Ferrara G. B., Cicimarra F., Buffolano W., Follo D., Auricchio S. Evidence that celiac disease is primarily associated with a DC locus allelic specificity. Clin Immunol Immunopathol. 1983 Sep;28(3):395–404. doi: 10.1016/0090-1229(83)90106-x. [DOI] [PubMed] [Google Scholar]
- Williamson N., Asquith P., Stokes L., Jowett W., Cooke W. T. Anticonnective tissue and other antitissue 'antibodies' in the sera of patients with coeliac disease compared with the findings in a mixed hospital population. J Clin Pathol. 1976 Jun;29(6):484–494. doi: 10.1136/jcp.29.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]


