Abstract
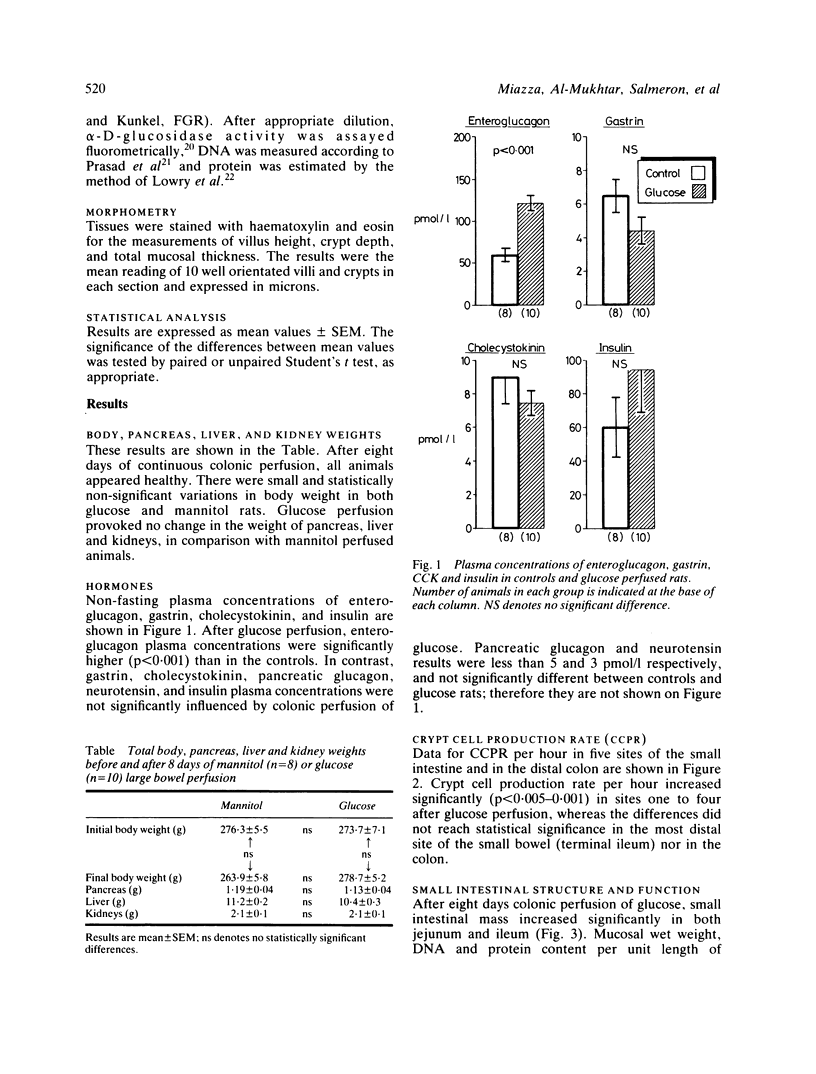
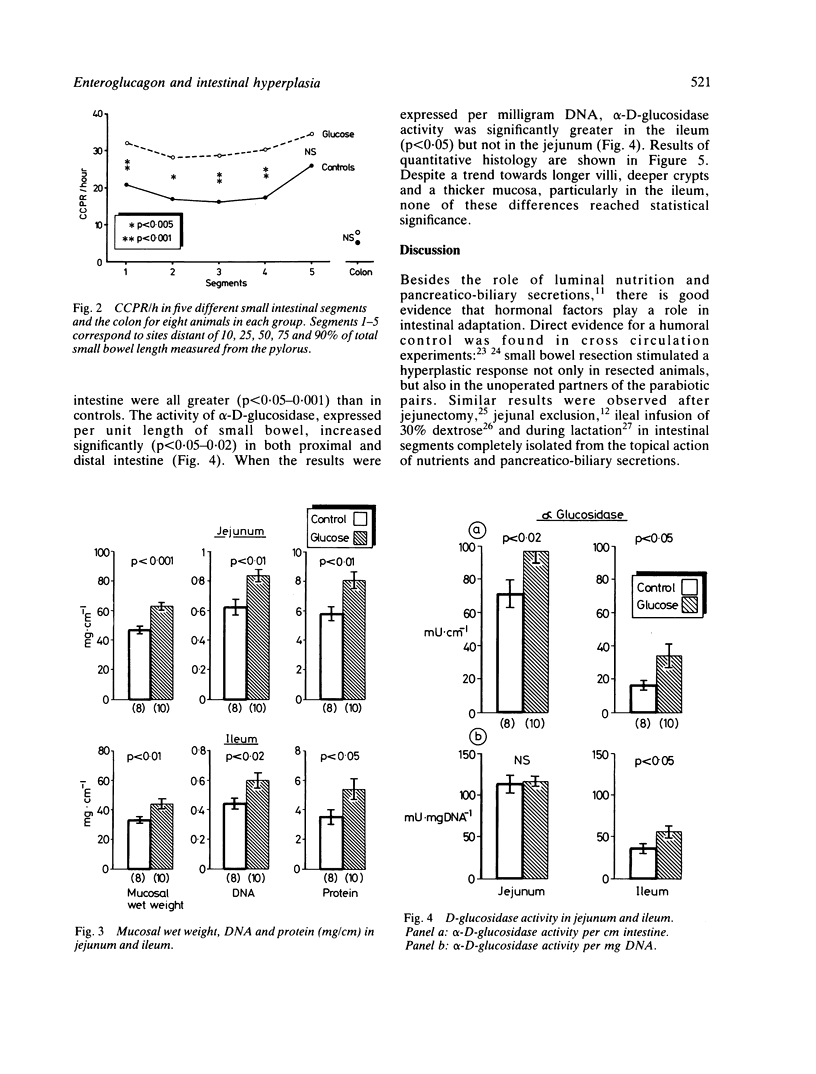
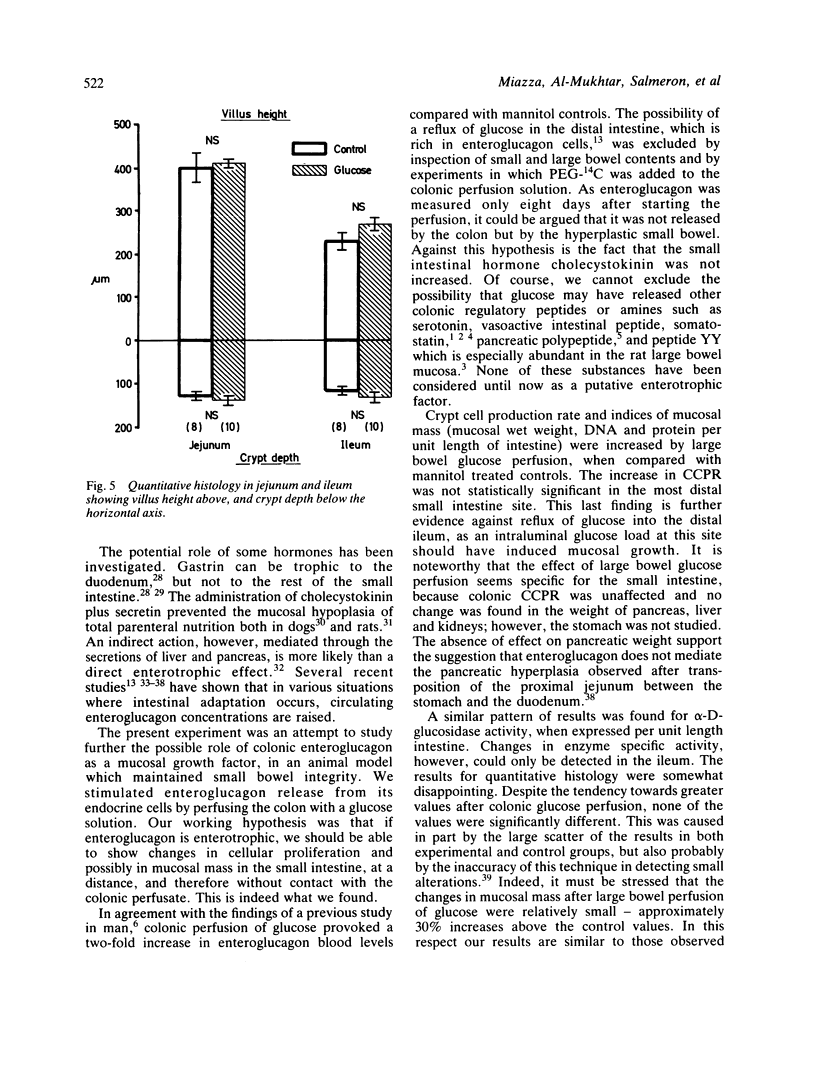
Beside intraluminal factors, humoral agents play an important role in intestinal adaptation. Enteroglucagon, the mucosal concentration of which is maximal in the terminal ileum and colon, is the strongest candidate for the role of small intestinal mucosal growth factor. The present experiment was designed to study the role of colonic enteroglucagon in stimulating mucosal growth in rats with a normal small intestine. After eight days of glucose large bowel perfusion, enteroglucagon plasma concentrations were 120.7 +/- SEM 9.2 pmol/l, versus 60.1 +/- 6.8 in mannitol perfused control rats (p less than 0.001). Gastrin, cholecystokinin, neurotensin, pancreatic glucagon, and insulin plasma concentrations were unchanged. Crypt cell proliferation, measured by the vincristine metaphase arrest technique, increased significantly in the small intestine of glucose perfused animals (p less than 0.005-0.001) in comparison with the controls. This resulted in a greater mucosal mass in both proximal and distal small bowel: mucosal wet weight, DNA, protein and alpha D-glucosidase per unit length intestine were all significantly higher (p less than 0.05-0.001) than in mannitol perfused rats. Our data, therefore, support the hypothesis that enteroglucagon is an enterotrophic factor and stress the possible role of the colon in the regulation of small bowel trophicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Blackburn A. M., Bloom S. R. A radioimmunoassay for neurotensin in human plasma. J Endocrinol. 1979 Nov;83(2):175–181. doi: 10.1677/joe.0.0830175. [DOI] [PubMed] [Google Scholar]
- Debas H. T. The colon as an endocrine organ. Dig Dis Sci. 1981 Mar;26(3):193–194. doi: 10.1007/BF01391628. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- El-Salhy M., Grimelius L., Wilander E., Ryberg B., Terenius L., Lundberg J. M., Tatemoto K. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77(1):15–23. doi: 10.1007/BF00496632. [DOI] [PubMed] [Google Scholar]
- Elias E., Dowling R. H. The mechanism for small-bowel adaptation in lactating rats. Clin Sci Mol Med. 1976 Nov;51(5):427–433. doi: 10.1042/cs0510427. [DOI] [PubMed] [Google Scholar]
- Ghatei M. A., Uttenthal L. O., Bryant M. G., Christofides N. D., Moody A. J., Bloom S. R. Molecular forms of glucagon-like immunoreactivity in porcine intestine and pancreas. Endocrinology. 1983 Mar;112(3):917–923. doi: 10.1210/endo-112-3-917. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Hood A. J., Mushens J., Smy J. R. Inhibition of external pancreatic secretion by intracolonic and intraileal infusions in the cat. J Physiol. 1979 Jul;292:445–454. doi: 10.1113/jphysiol.1979.sp012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Bates T., Dowling R. H. Cholecystokinin and secretin prevent the intestinal mucosal hypoplasia of total parenteral nutrition in the dog. Gastroenterology. 1978 Jul;75(1):34–41. [PubMed] [Google Scholar]
- Höfler H., Kasper M., Heitz P. U. The neuroendocrine system of normal human appendix, ileum and colon, and in neurogenic appendicopathy. Virchows Arch A Pathol Anat Histopathol. 1983;399(2):127–140. doi: 10.1007/BF00619574. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Bloom S. R., Dowling R. H. Response of plasma and tissue levels of enteroglucagon immunoreactivity to intestinal resection, lactation and hyperphagia. Life Sci. 1981 Nov 9;29(19):2003–2007. doi: 10.1016/0024-3205(81)90610-x. [DOI] [PubMed] [Google Scholar]
- Jian R., Besterman H. S., Sarson D. L., Aymes C., Hostein J., Bloom S. R., Rambaud J. C. Colonic inhibition of gastric secretion in man. Dig Dis Sci. 1981 Mar;26(3):195–201. doi: 10.1007/BF01391629. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Lichtenberger L. M., Copeland E. M., Dudrick S. J., Castro G. A. Action of gastrin on gastrointestinal structure and function. Gastroenterology. 1975 May;68(5 Pt 1):1184–1192. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson L. I. Gastrointestinal cells producing endocrine, neurocrine and paracrine messengers. Clin Gastroenterol. 1980 Sep;9(3):485–416. [PubMed] [Google Scholar]
- Lundberg J. M., Tatemoto K., Terenius L., Hellström P. M., Mutt V., Hökfelt T., Hamberger B. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4471–4475. doi: 10.1073/pnas.79.14.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge H., Gräfe M., Lorenz-Meyer H., Riecken E. O. The influence of food intake on the development of structural and functional adaptation following ileal resection in the rat. Gut. 1975 Jun;16(6):468–472. doi: 10.1136/gut.16.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarson J. E., Veen H. F., Williamson R. C., Ross J. S., Malt R. A. Compensatory postresectional hyperplasia and starvation atrophy in small bowel: dissociation from endogenous gastrin levels. Gastroenterology. 1977 May;72(5 Pt 1):890–895. [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., DuMouchelle E., Koniuch D., Oberleas D. A simple fluorometric method for the determination of RNA and DNA in tissues. J Lab Clin Med. 1972 Oct;80(4):598–602. [PubMed] [Google Scholar]
- Sagor G. R., Al-Mukhtar M. Y., Ghatei M. A., Wright N. A., Bloom S. R. The effect of altered luminal nutrition on cellular proliferation and plasma concentrations of enteroglucagon and gastrin after small bowel resection in the rat. Br J Surg. 1982 Jan;69(1):14–18. doi: 10.1002/bjs.1800690106. [DOI] [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Evidence for a humoral mechanism after small intestinal resection. Exclusion of gastrin but not enteroglucagon. Gastroenterology. 1983 May;84(5 Pt 1):902–906. [PubMed] [Google Scholar]
- Seal A. M., Debas H. T. Colonic inhibition of gastric acid secretion in the dog. Gastroenterology. 1980 Nov;79(5 Pt 1):823–826. [PubMed] [Google Scholar]
- Spector M. H., Levine G. M., Deren J. J. Direct and indirect effects of dextrose and amino acids on gut mass. Gastroenterology. 1977 Apr;72(4 Pt 1):706–710. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L. Evidence for an enterotropic hormone: compensatory hyperplasia in defunctioned bowel. Br J Surg. 1978 Oct;65(10):736–739. doi: 10.1002/bjs.1800651018. [DOI] [PubMed] [Google Scholar]
- Williamson R. C., Buchholtz T. W., Malt R. A. Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology. 1978 Aug;75(2):249–254. [PubMed] [Google Scholar]
- Wright N. A., Appleton D. R. The metaphase arrest technique. A critical review. Cell Tissue Kinet. 1980 Nov;13(6):643–663. doi: 10.1111/j.1365-2184.1980.tb00503.x. [DOI] [PubMed] [Google Scholar]


