Abstract
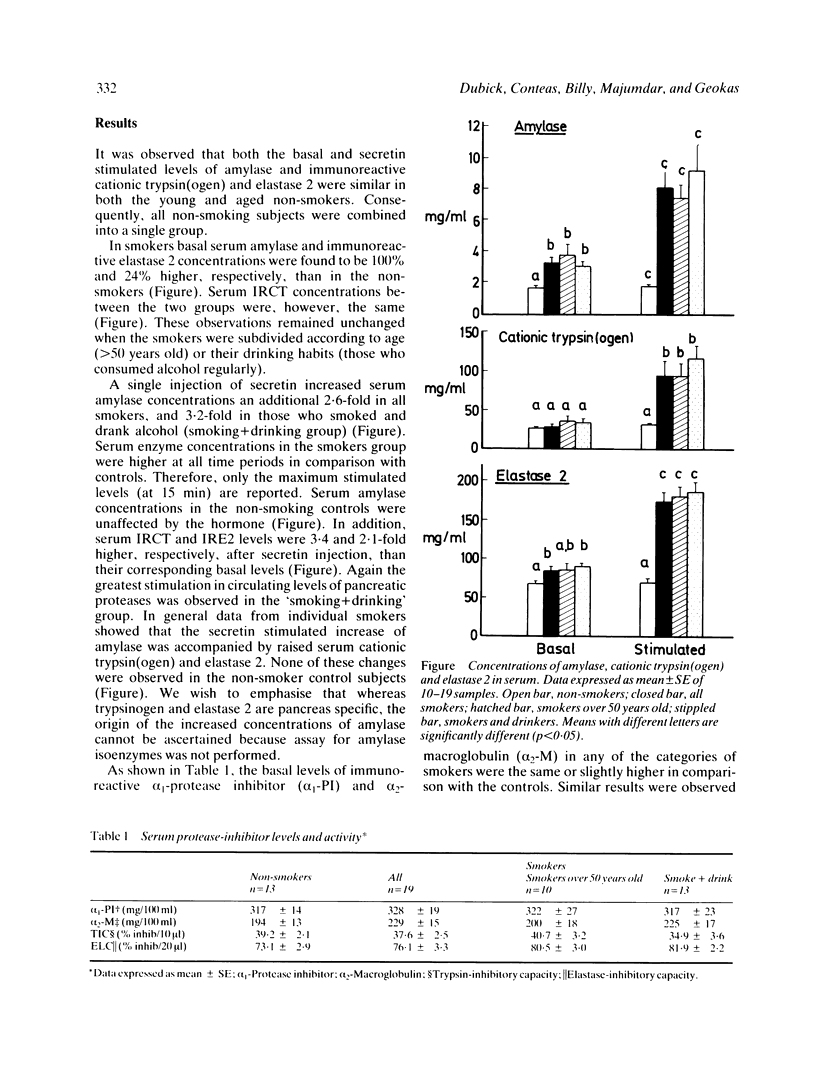
Circulating concentrations of digestive enzymes, certain lysosomal hydrolases and protease inhibitors were measured in 19 heavy smokers and 13 non-smokers before (basal) and at 15, 30, and 60 minutes after a single intravenous injection of secretin (75 CU). In smokers, basal serum amylase and immunoreactive pancreatic elastase 2 (IRE2) concentrations were about 100% and 25% higher respectively, than in the non-smokers, whereas, no differences were observed in basal immunoreactive cationic trypsinogen (IRCT) concentrations and in acid phosphatase and beta-glucuronidase activities between the two groups. Furthermore, a single injection of secretin to cigarette smokers significantly increased serum amylase, IRCT and IRE2 by 155%, 200%, and 100%, respectively when compared with their corresponding basal levels. No such increment was observed in the non-smokers. In addition, there were no significant differences in serum trypsin or elastase inhibitory capacity or immunoreactive alpha 1-protease inhibitor and alpha 2-macroglobulin levels between smokers and non-smokers. The levels and inhibitory capacity of these protease inhibitors was also not affected by secretin injection. These data suggest that cigarette smoking enhances the responsiveness of the exocrine pancreas to a physiological stimulus such as secretin, with resultant substantial increase in the concentrations of pancreatic hydrolases in blood.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud R. T., Fera T., Richter A., Tabona M. Z., Johal S. Acute effect of smoking on the functional activity of alpha1-protease inhibitor in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1985 Jan;131(1):79–85. doi: 10.1164/arrd.1985.131.1.79. [DOI] [PubMed] [Google Scholar]
- Andriulli A., Masoero G., Amato A., Felder M., Benitti V., Dobrilla G., De La Pierre M., Verme G. Serum immunoreactive cationic trypsinogen response to secretin in normal subjects. Am J Gastroenterol. 1983 Sep;78(9):579–583. [PubMed] [Google Scholar]
- Auerbach O., Garfinkel L. Atherosclerosis and aneurysm of aorta in relation to smoking habits and age. Chest. 1980 Dec;78(6):805–809. doi: 10.1378/chest.78.6.805. [DOI] [PubMed] [Google Scholar]
- BURTON P., HAMMOND E. M., HARPER A. A., HOWAT H. T., SCOTT J. E., VARLEY H. Serum amylase and serum lipase levels in man after administration of secretin and pancreozymin. Gut. 1960 Jun;1:125–139. doi: 10.1136/gut.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balart L. A., Ferrante W. A. Pathophysiology of acute and chronic pancreatitis. Arch Intern Med. 1982 Jan;142(1):113–117. [PubMed] [Google Scholar]
- Balldin G., Borgström A., Eddeland A., Genell S., Hagberg L., Ohlsson K. Elevated serum levels of pancreatic secretory proteins in cigarette smokers after secretin stimulation. J Clin Invest. 1980 Jul;66(1):159–162. doi: 10.1172/JCI109829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty K., Robertie P., Senior R. M., Travis J. Determination of oxidized alpha-1-proteinase inhibitor in serum. J Lab Clin Med. 1982 Aug;100(2):186–192. [PubMed] [Google Scholar]
- Busuttil R. W., Rinderbriecht H., Flesher A., Carmack C. Elastase activity: the role of elastase in aortic aneurysm formation. J Surg Res. 1982 Mar;32(3):214–217. doi: 10.1016/0022-4804(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Cannon D. J., Read R. C. Blood elastolytic activity in patients with aortic aneurysm. Ann Thorac Surg. 1982 Jul;34(1):10–15. doi: 10.1016/s0003-4975(10)60845-4. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Carr-Locke D. L., Gregg J. A., Chey W. Y. Effects of exogenous secretin on pancreatic and biliary ductal and sphincteric pressures in man demonstrated by endoscopic manometry and correlation with plasma secretin levels. Dig Dis Sci. 1985 Oct;30(10):909–917. doi: 10.1007/BF01308289. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Hendricks J. C., Dozois R. R., Go V. L. Effect of secretin on pancreatic duct pressure, resistance to pancreatic flow, and duodenal motor activity in the dog. Dig Dis Sci. 1981 Jan;26(1):1–6. doi: 10.1007/BF01307969. [DOI] [PubMed] [Google Scholar]
- Fried G. M., Ogden W. D., Zhu X. G., Greeley G. H., Jr, Thompson J. C. Effect of alcohol on the release of cholecystokinin and pancreatic enzyme secretion. Am J Surg. 1984 Jan;147(1):53–57. doi: 10.1016/0002-9610(84)90034-5. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Brodrick J. W., Johnson J. H., Largman C. Pancreatic elastase in human serum. Determination by radioimmunoassay. J Biol Chem. 1977 Jan 10;252(1):61–67. [PubMed] [Google Scholar]
- Geokas M. C. Ethanol and the pancreas. Med Clin North Am. 1984 Jan;68(1):57–75. doi: 10.1016/s0025-7125(16)31241-x. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Largman C., Brodrick J. W., Fassett M. Molecular forms of immunoreactive pancreatic elastase in canine pancreatic and peripheral blood. Am J Physiol. 1980 Mar;238(3):G238–G246. doi: 10.1152/ajpgi.1980.238.3.G238. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Largman C., Brodrick J. W., Johnson J. H. Determination of human pancreatic cationic trypsinogen in serum by radioimmunoassay. Am J Physiol. 1979 Jan;236(1):E77–E83. doi: 10.1152/ajpendo.1979.236.1.E77. [DOI] [PubMed] [Google Scholar]
- Gullo L., Priori P., Costa P. L., Mattioli G., Labò G. Action of secretin on pancreatic enzyme secretion in man. Studies on pure pancreatic juice. Gut. 1984 Aug;25(8):867–873. doi: 10.1136/gut.25.8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz A. J., Anderson A. J., Brooks H. L., Manley J. C., Parent G. T., Barboriak J. J. The association of smoking with cardiomyopathy. N Engl J Med. 1984 Nov 8;311(19):1201–1206. doi: 10.1056/NEJM198411083111901. [DOI] [PubMed] [Google Scholar]
- Hill P., Haley N. J., Wynder E. L. Cigarette smoking: carboxyhemoglobin, plasma nicotine, cotinine and thiocyanate vs self-reported smoking data and cardiovascular disease. J Chronic Dis. 1983;36(6):439–449. doi: 10.1016/0021-9681(83)90136-4. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H., Laurent P., Raju L. The role of oxidative processes in emphysema. Am Rev Respir Dis. 1983 Feb;127(2):S31–S38. doi: 10.1164/arrd.1983.127.2P2.S31. [DOI] [PubMed] [Google Scholar]
- Jung D. H. Preparation and application of Procion Yellow starch for amylase assay. Clin Chim Acta. 1980 Jan 1;100(1):7–11. doi: 10.1016/0009-8981(80)90179-5. [DOI] [PubMed] [Google Scholar]
- Kaufman D. W., Helmrich S. P., Rosenberg L., Miettinen O. S., Shapiro S. Nicotine and carbon monoxide content of cigarette smoke and the risk of myocardial infarction in young men. N Engl J Med. 1983 Feb 24;308(8):409–413. doi: 10.1056/NEJM198302243080801. [DOI] [PubMed] [Google Scholar]
- Largman C., Brodrick J. W., Geokas M. C. Radioimmunoassay determination of circulating pancreatic endopeptidases. Methods Enzymol. 1981;74(Pt 100):272–290. doi: 10.1016/0076-6879(81)74019-9. [DOI] [PubMed] [Google Scholar]
- Majumdar A. P., Davis G. A., Dubick M. A., Geokas M. C. Nicotine stimulation of protein secretion from isolated rat pancreatic acini. Am J Physiol. 1985 Feb;248(2 Pt 1):G158–G163. doi: 10.1152/ajpgi.1985.248.2.G158. [DOI] [PubMed] [Google Scholar]
- Majumdar A. P., Vesenka G. D., Dubick M. A., Yu G. S., DeMorrow J. M., Geokas M. C. Morphological and biochemical changes of the pancreas in rats treated with acetaldehyde. Am J Physiol. 1986 May;250(5 Pt 1):G598–G606. doi: 10.1152/ajpgi.1986.250.5.G598. [DOI] [PubMed] [Google Scholar]
- Mayer M., Yedgar S., Joffe M., Shafrir E. Urine protease and antiprotease activity in experimental aminonucleoside nephrotoxicity. Nephron. 1981;29(5-6):223–228. doi: 10.1159/000182376. [DOI] [PubMed] [Google Scholar]
- Murthy S. N., Dinoso V. P., Jr, Clearfield H. R., Chey W. Y. Simultaneous measurement of basal pancreatic, gastric acid secretion, plasma gastrin, and secretin during smoking. Gastroenterology. 1977 Oct;73(4 Pt 1):758–761. [PubMed] [Google Scholar]
- Rinderknecht H., Stace N. H., Renner I. G. Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Dig Dis Sci. 1985 Jan;30(1):65–71. doi: 10.1007/BF01318373. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Lewin M. B., Wong A., Deveney C. W., Wendland M. F., Leimgruber R. M., Geokas M. C. Irreversible inhibition by acetaldehyde of cholecystokinin-induced amylase secretion from isolated rat pancreatic acini. Biochem Pharmacol. 1985 Aug 15;34(16):2859–2863. doi: 10.1016/0006-2952(85)90007-3. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Calore J. D., McGowan S. E., Bernardo J., Snider G. L., Franzblau C. Functional alpha 1-protease inhibitor in the lower respiratory tract of cigarette smokers is not decreased. Science. 1983 Sep 16;221(4616):1187–1189. doi: 10.1126/science.6612333. [DOI] [PubMed] [Google Scholar]
- Uhlemann E. R., Robberecht P., Gardner J. D. Effects of alcohols on the actions of VIP and secretin on acinar cells from guinea pig pancreas. Gastroenterology. 1979 May;76(5 Pt 1):917–925. [PubMed] [Google Scholar]
- Walberg C. B., Geokas M. C., Rinderknecht H. Determination of serum methemalbumin. Clin Chim Acta. 1973 Oct 12;48(2):229–232. doi: 10.1016/0009-8981(73)90370-7. [DOI] [PubMed] [Google Scholar]
- Yen S., Hsieh C. C., MacMahon B. Consumption of alcohol and tobacco and other risk factors for pancreatitis. Am J Epidemiol. 1982 Sep;116(3):407–414. doi: 10.1093/oxfordjournals.aje.a113425. [DOI] [PubMed] [Google Scholar]


