Abstract
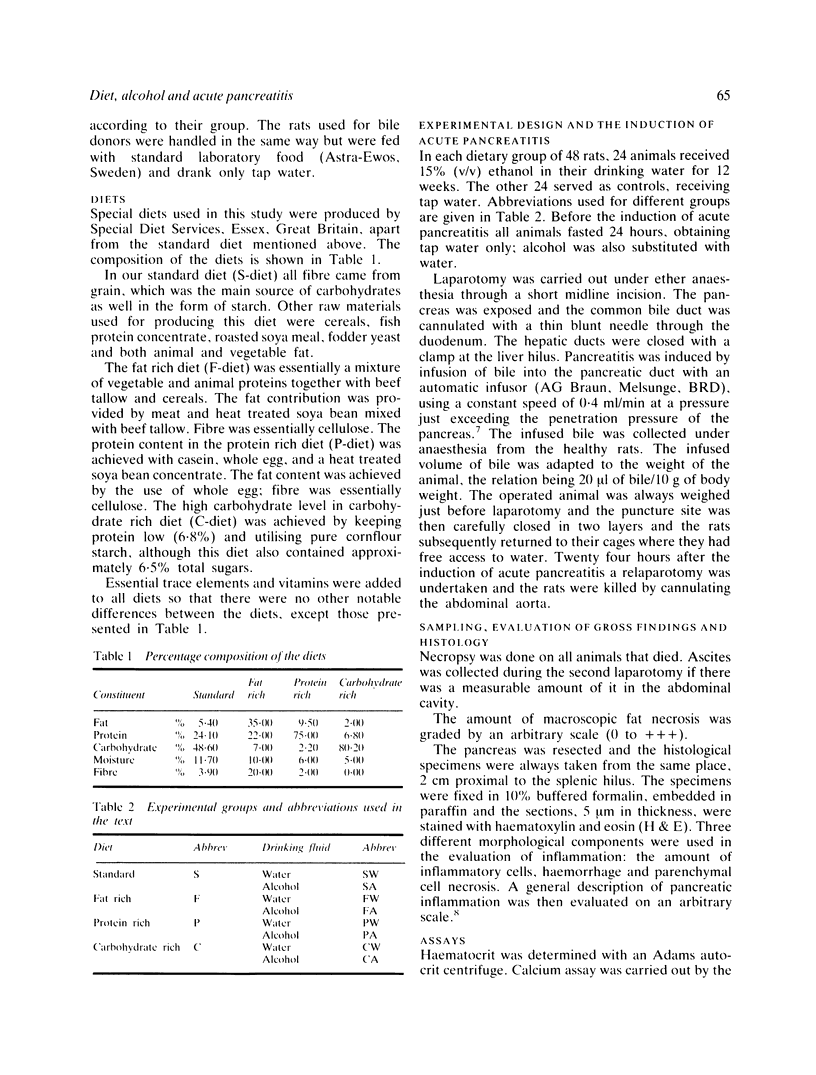
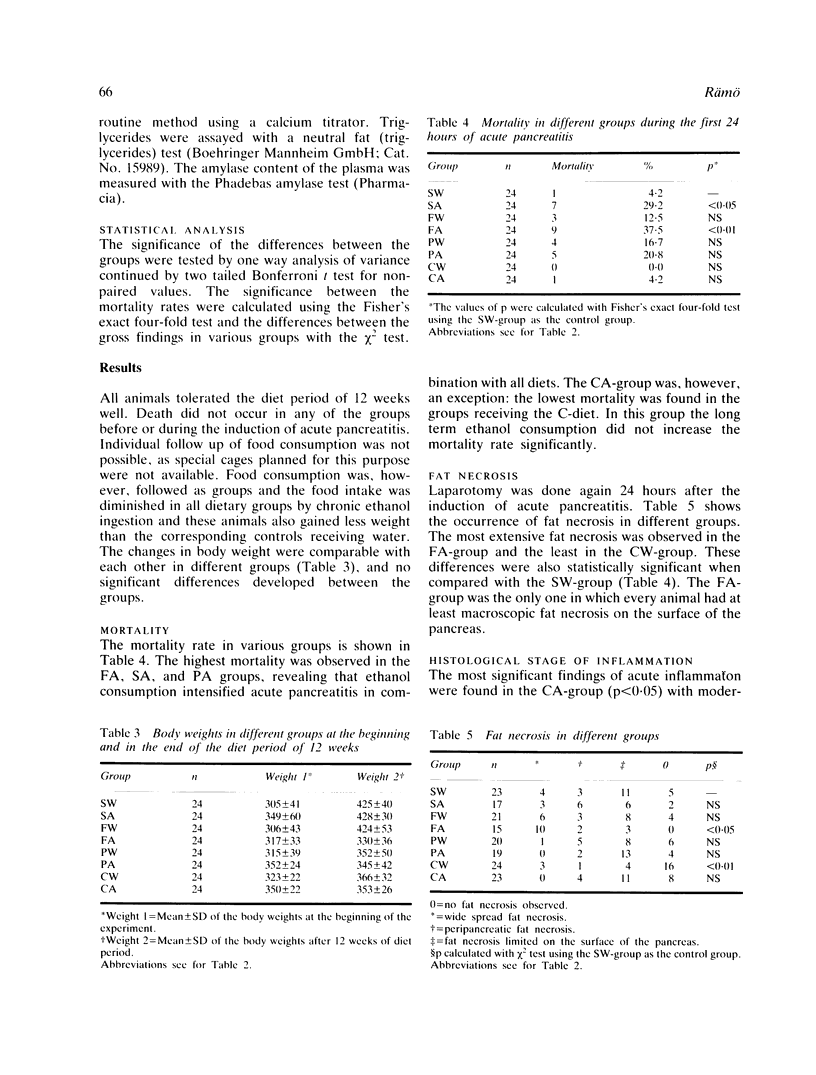
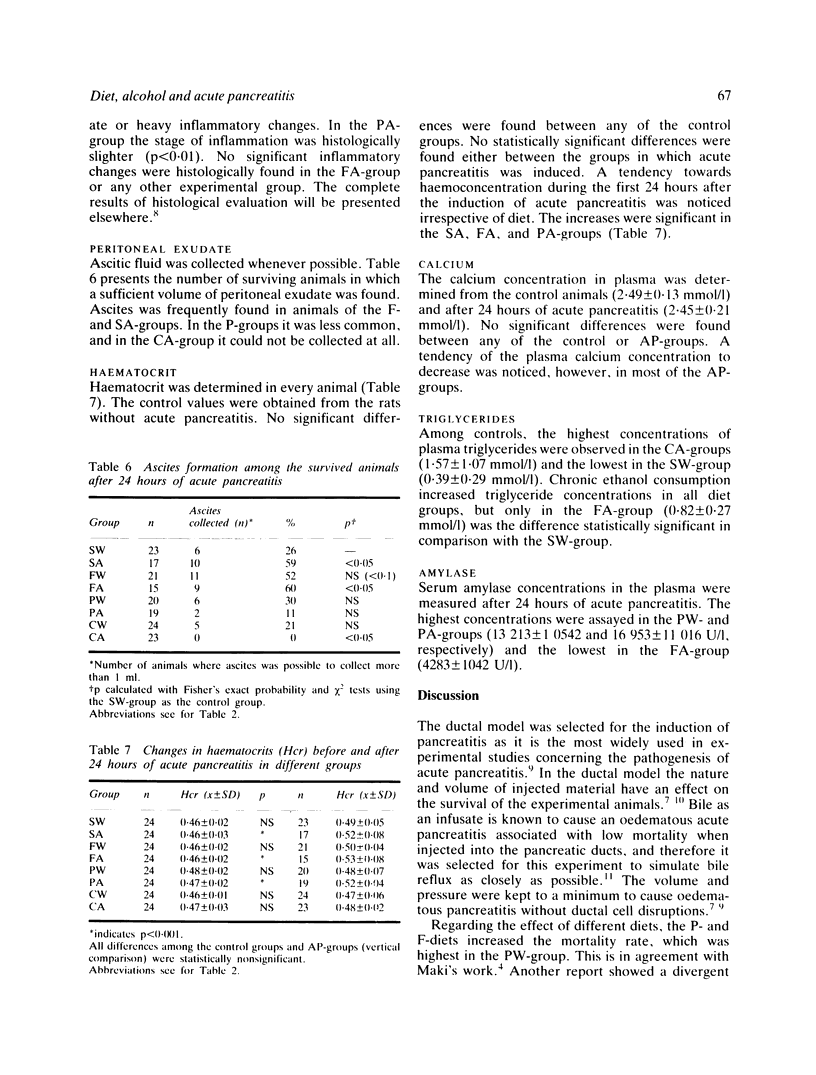
The effect of fat rich (F), protein rich (P) and carbohydrate rich (C) diets and chronic ethanol consumption on experimental acute pancreatitis was studied in rats. One hundred and ninety two animals with induced acute pancreatitis were divided into eight groups fed either a mixture of water and 15% ethanol (v/v), or tap water combined with standard or special diets according to their group, for 12 weeks. The other control 192 rats were divided into equal groups. Bile induced experimental acute pancreatitis caused the highest mortality (37.5%) in the animals receiving F diet and ethanol. In this group significant haemoconcentration, peritoneal exudate formation and the most extensive fat necrosis were also observed. The carbohydrate rich diet with or without ethanol did not have any significant effect on the severity of acute pancreatitis. Diet and ethanol may alter the metabolism of the pancreas and cause derangements at the systemic level. These derangements might cause the increased susceptibility to acute pancreatitis. These changes in the metabolism may be fatal because of the increased toxicity of the peritoneal exudate secreted during the inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho H. J., Suonpä K., Ahola R. A., Nevalainen T. J. Experimental pancreatitis in the rat. Ductal factors in sodium taurocholate-induced acute pancreatitis. Exp Pathol. 1984;25(2):73–79. doi: 10.1016/s0232-1513(84)80010-9. [DOI] [PubMed] [Google Scholar]
- Brian Haig T. H. Experimental pancreatitis intensified by a high fat diet. Surg Gynecol Obstet. 1970 Nov;131(5):914–918. [PubMed] [Google Scholar]
- Cameron J. L., Crisler C., Margolis S., DeMeester T. R., Zuidema G. D. Acute pancreatitis with hyperlipemia. Surgery. 1971 Jul;70(1):53–61. [PubMed] [Google Scholar]
- Durbec J. P., Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18(5-6):337–350. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- ELLIOTT D. W., WILLIAMS R. D., ZOLLINGER R. M. Alterations in the pancreatic resistance to bile in the pathogenesis of acute pancreatitis. Ann Surg. 1957 Oct;146(4):669–682. doi: 10.1097/00000658-195710000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. A., Llach F., Massry S. G. Acute renal failure in patients with acute pancreatitis. Arch Intern Med. 1976 Dec;136(12):1363–1365. [PubMed] [Google Scholar]
- Goslin J., Hong S. S., Magee D. F., White T. T. Relationship between diet, ethyl alcohol consumption and some activities of the exocrine pancreas in rats. Arch Int Pharmacodyn Ther. 1965 Oct;157(2):462–469. [PubMed] [Google Scholar]
- Jalovaara P., Apaja M. Alcohol and acute pancreatitis. An experimental study in the rat. Scand J Gastroenterol. 1978;13(6):703–709. doi: 10.3109/00365527809181784. [DOI] [PubMed] [Google Scholar]
- Kanayama R., Takase S., Matsuda Y., Takada A. Effect of dietary fat upon ethanol metabolism in rats. Biochem Pharmacol. 1984 Oct 15;33(20):3283–3287. doi: 10.1016/0006-2952(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Koop H., Winckler K., Fölsch U. R., Creutzfeldt W. Somatostatin therapy of acute experimental pancreatitis. Gut. 1977 Sep;18(9):713–716. doi: 10.1136/gut.18.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T., Kakizaki G., Sato T., Saito Y., Suda Y. Effect of diet on experimental pancreatitis in rat. Tohoku J Exp Med. 1967 Jul;92(3):301–309. doi: 10.1620/tjem.92.301. [DOI] [PubMed] [Google Scholar]
- Satake K., Koh I., Nishiwaki H., Umeyama K. Toxic products in hemorrhagic ascitic fluid generated during experimental acute hemorrhagic pancreatitis in dogs and a treatment which reduces their effect. Digestion. 1985;32(2):99–105. doi: 10.1159/000199225. [DOI] [PubMed] [Google Scholar]
- Satake K., Rozmanith J. S., Appert H. E., Carballo J., Howard J. M. Hypotension and release of kinin-forming enzyme into ascitic fluid exudate during experimental pancreatitis in dogs. Ann Surg. 1973 Apr;177(4):497–502. doi: 10.1097/00000658-197304000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzhilin D. A., Dreiling D. A. Cardiovascular lesions in pancreatitis. Am J Gastroenterol. 1975 May;63(5):381–388. [PubMed] [Google Scholar]
- Wilson J. S., Pirola R. C. Pathogenesis of alcoholic pancreatitis. Aust N Z J Med. 1983 Jun;13(3):307–312. doi: 10.1111/j.1445-5994.1983.tb04672.x. [DOI] [PubMed] [Google Scholar]


