Abstract
The effect of low dose warfarin and high dose warfarin on epithelial cell kinetics (as determined by stathmokinetic techniques), and preneoplastic morphological changes was studied during azoxymethane induced carcinogenesis in the rat. Warfarin, at either low or high dose, had no effect on crypt cell production rate (CCPR) at any time interval whereas tumour incidence in both low dose warfarin and high dose warfarin groups was significantly reduced. Morphological changes were observed using scanning electron microscopy, which by conventional histology were shown to be adenoma precursors. In the control group the number of microadenomas increased with time after starting azoxymethane. In warfarin treated animals, the number of microadenomas also increased with time, but the actual incidence was reduced when compared with controls. These results suggest that the effects of warfarin on tumour development is unrelated to its anticoagulant effect, because increased dose did not result in greater tumour reduction. Furthermore, there was no overall change in CCPR when warfarin was administered. Warfarin may exert a specific effect, by preventing neoplastic change in cells which have undergone morphologically undetectable changes associated with early carcinogenesis.
Full text
PDF
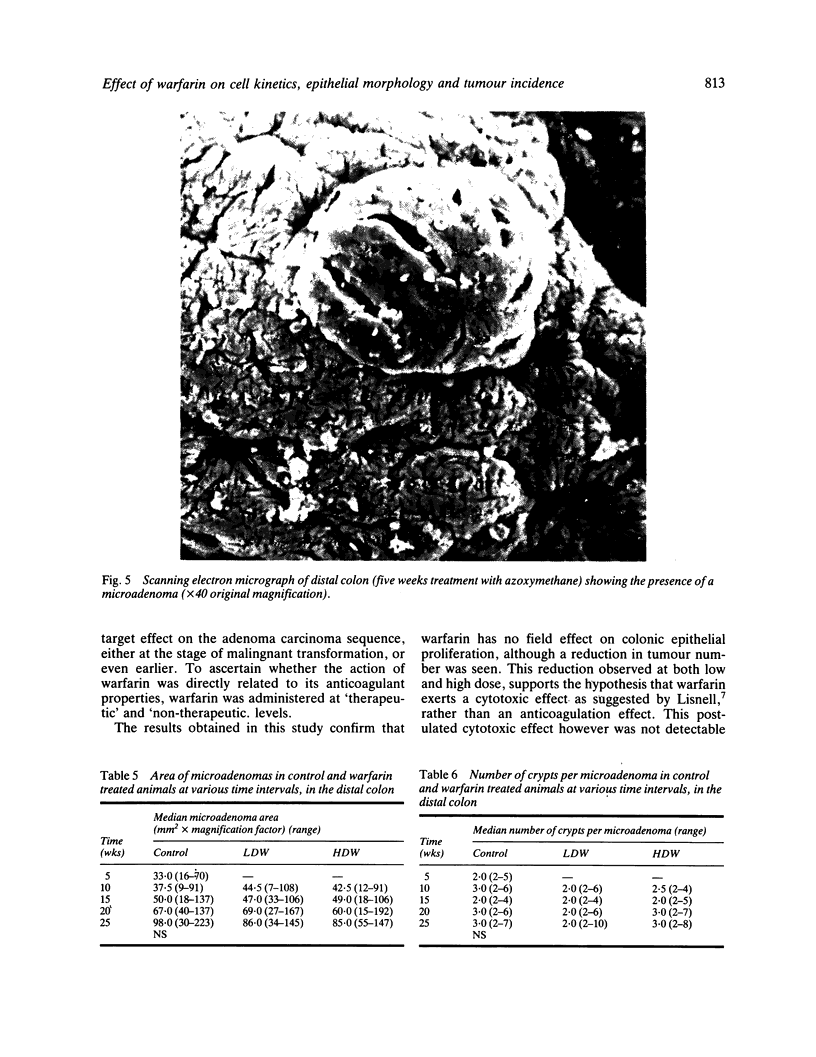


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkla D. H., Tutton P. J. Surface changes in the descending colon of rats treated with dimethylhydrazine. Cancer Res. 1977 Jan;37(1):262–271. [PubMed] [Google Scholar]
- Brown J. M. A study of the mechanism by which anticoagulation with warfarin inhibits blood-borne metastases. Cancer Res. 1973 Jun;33(6):1217–1224. [PubMed] [Google Scholar]
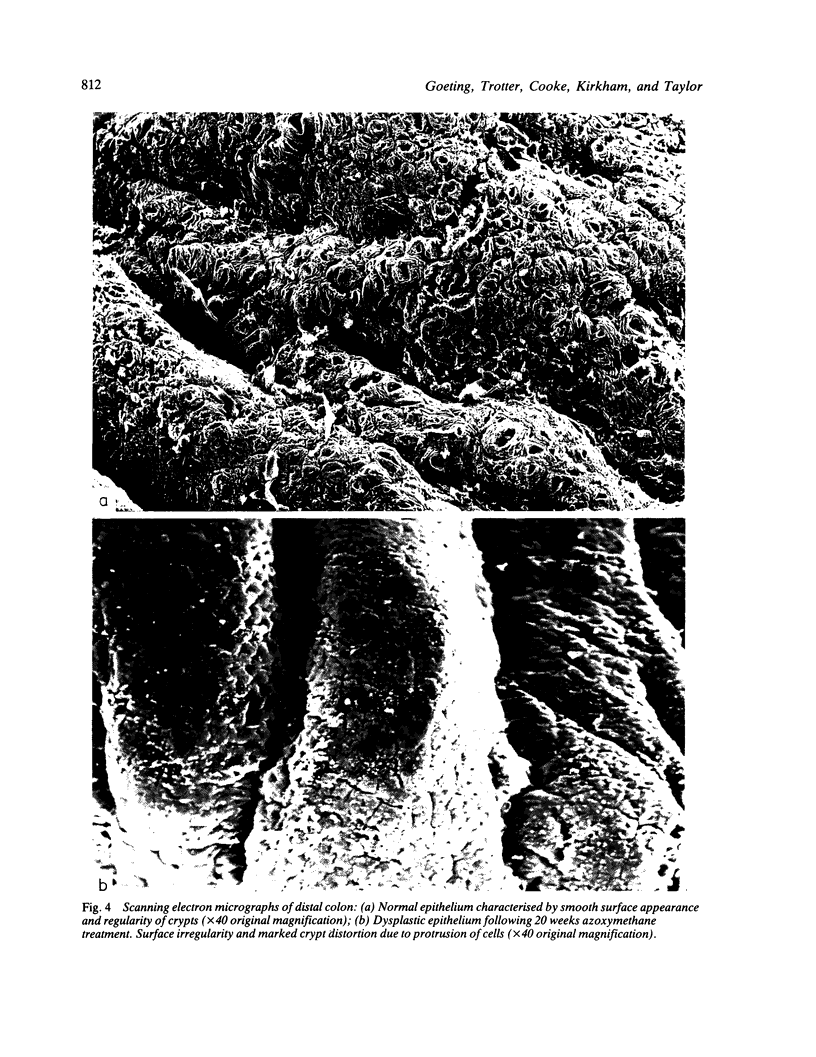
- Cooke T., Kirkham N., Stainthorp D. H., Inman C., Goeting N., Taylor I. Detection of early neoplastic changes in experimentally induced colorectal cancer using scanning electron microscopy and cell kinetic studies. Gut. 1984 Jul;25(7):748–755. doi: 10.1136/gut.25.7.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISNELL A., MELLGREN J. Effect of heparin, protamine, dicoumarol, streptokinase and epsilon-amino-n-caproic acid on the growth of human cells in vitro. Acta Pathol Microbiol Scand. 1963;57:145–153. doi: 10.1111/j.1699-0463.1963.tb03439.x. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., O'Gorman T. A. Experimental colon cancer. Gastroenterology. 1978 Dec;75(6):1157–1169. [PubMed] [Google Scholar]
- Mooney B., Serlin M., Taylor I. The effect of warfarin on spontaneously metastasising colorectal cancer in the rat. Clin Oncol. 1982 Mar;8(1):55–59. [PubMed] [Google Scholar]
- Ryan J. J., Ketcham A. S., Wexler H. Warfarin treatment of mice bearing autochthonous tumors: effect on spontaneous metastases. Science. 1968 Dec 27;162(3861):1493–1494. doi: 10.1126/science.162.3861.1493. [DOI] [PubMed] [Google Scholar]
- Traynor O. J., Costa N. L., Blumgart L. H., Wood C. B. A scanning electron microscopy study of ultrastructural changes in the colonic mucosa of patients with large bowel tumours. Br J Surg. 1981 Oct;68(10):701–704. doi: 10.1002/bjs.1800681010. [DOI] [PubMed] [Google Scholar]
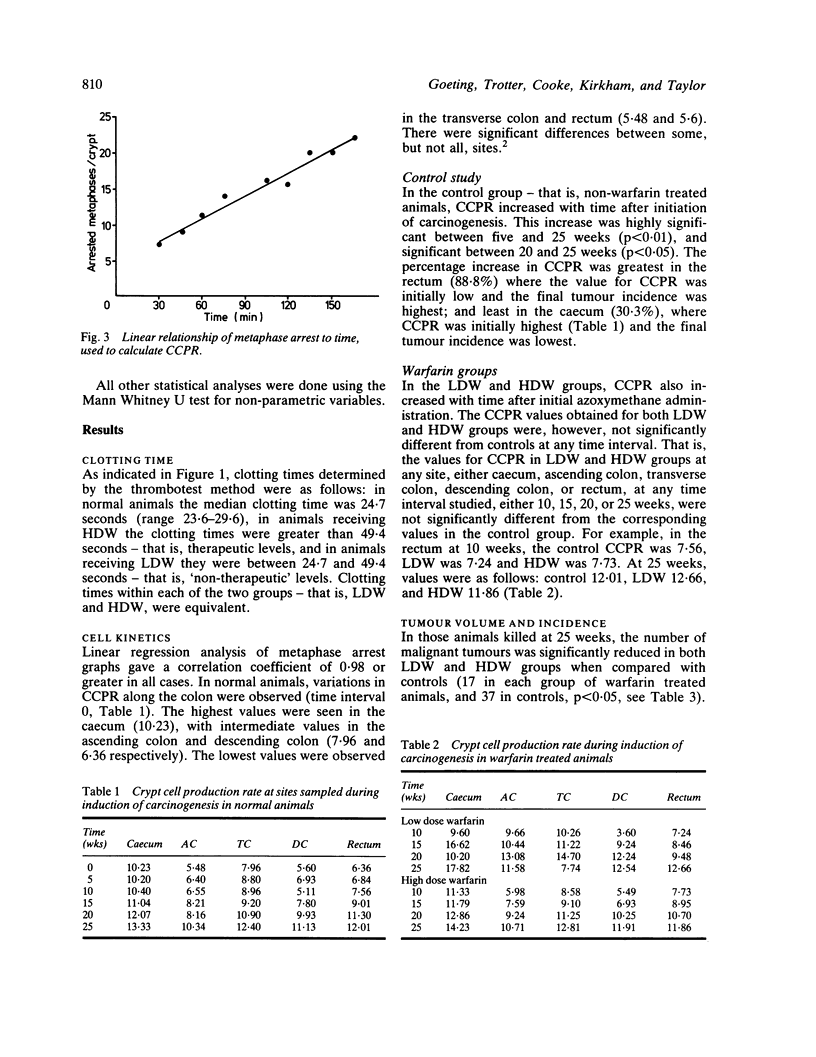
- Wright N. A., Appleton D. R. The metaphase arrest technique. A critical review. Cell Tissue Kinet. 1980 Nov;13(6):643–663. doi: 10.1111/j.1365-2184.1980.tb00503.x. [DOI] [PubMed] [Google Scholar]