Recent studies on the molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced enterocolitis using tissue culture models and the neonatal calf model have led to an improved understanding of key events occurring during the complex series of host-pathogen interactions leading to diarrhea. In vitro studies show that Salmonella serovar Typhimurium translocates a number of type III secreted effector proteins, including SipA, SopA, SopB, SopD, and SopE2 into host cells. SipA, SopA, SopB, SopD, and SopE2 are required for eliciting infiltration of neutrophils in the calf model of enterocolitis, presumably by inducing the production of chemoattractant chemokines in bovine ileal tissue during Salmonella serovar Typhimurium infection. The resulting acute inflammatory response is associated with an increase in vascular permeability resulting in mucosal edema. Furthermore, the influx of neutrophils is associated with necrosis of the uppermost ileal mucosa. The injury to the intestinal epithelium leads to leakage of extravascular fluids and massive transmigration of neutrophils into the intestinal lumen, a process normally prevented by the epithelial permeability barrier. These data suggest that the severe fluid loss observed during Salmonella serovar Typhimurium-induced enterocolitis is at least in part due to an inflammatory mechanism which causes liquid to flow from the blood to the intestinal lumen.
THE IMPORTANCE IN THE UNITED STATES OF HUMAN DISEASE SYNDROMES CAUSED BY SALMONELLA SEROTYPES
Salmonella serotypes are associated with three distinct human disease syndromes, bacteremia, typhoid fever, and enterocolitis. Of these, bacteremia, a syndrome caused by the porcine-adapted S. enterica serotype Choleraesuis and the bovine-adapted S. enterica serotype Dublin, is encountered least frequently in humans (20, 88). Typhoid fever, which is caused by the human-adapted S. enterica serotype Typhi, is essentially eradicated from the United States, although foreign travelers returning from areas of endemicity in Asia, Africa, or South America import approximately 800 cases annually, resulting in an estimated 3 deaths (66). In contrast, enterocolitis is the second most frequent cause of bacterial food-borne disease of known etiology in the United States, with an estimated 1.4 million illnesses per year (66). Furthermore, with approximately 550 annual deaths, Salmonella-induced enterocolitis is the single most common cause of death from food-borne illnesses associated with viruses, parasites, or bacteria in the United States (66). The serotype associated most frequently with this diarrheal disease syndrome in the United States is Salmonella serotype Typhimurium, accounting for 26% of all Salmonella isolates reported to the Centers for Disease Control (CDC) in 1998 (10).
THE USE OF ANIMAL MODELS TO STUDY ENTEROCOLITIS
Most studies on Salmonella pathogenesis elucidate virulence mechanisms using a typhoid fever model, namely Salmonella serotype Typhimurium infection in mice. Salmonella serotype Typhimurium causes a typhoid fever-like disease in mice, with intestinal and extraintestinal lesions closely resembling those observed in typhoid fever victims (56, 58, 74, 76). One reason for the popularity of this model may be that typhoid fever can be induced experimentally only by oral infection of humans or higher primates (e.g., chimpanzees), whereas lower primates (e.g., rhesus monkeys) or nonprimate vertebrates are resistant to infection with Salmonella serotype Typhi (30). However, while Salmonella serotype Typhimurium infection of mice results in a typhoid fever-like disease, this pathogen is associated exclusively with enterocolitis in humans. Thus, mice and humans exhibit strikingly different disease syndromes and host responses after infection with Salmonella serotype Typhimurium.
Mice infected with Salmonella serotype Typhimurium have signs of disease (i.e., elevated temperature as indicated by ruffled fur) between 4 and 8 days after oral infection but do not develop diarrhea. The long incubation period and the signs of disease in mice parallel the development of typhoid fever in volunteers. In volunteers infected with Salmonella serotype Typhi, fever manifests as the first symptom after a median incubation period of 5 to 9 days, depending on the challenge dose (42). Diarrhea is considered to be an insignificant symptom that develops late in infection (subsequent to the onset of fever) and in only one-third of typhoid fever patients (68). In contrast, volunteers infected with Salmonella serotype Typhimurium develop a clinical illness characterized by diarrhea, vomiting, and abdominal pain after an incubation period of only 12 to 72 h (7). In mice, Salmonella serotype Typhimurium spreads systemically via the circulation and the infection is characterized by severe pathological changes and high bacterial tissue load in Peyer's patches, mesenteric lymph nodes, the liver, and the spleen (74, 76). A similar systemic distribution of bacteria in tissues has been reported for typhoid fever patients (6, 56), and a low-level bacteremia is typically detected in this syndrome (9, 43). In contrast, Salmonella serotype Typhimurium infection in humans usually remains localized to the intestine and mesenteric lymph nodes, while bacteremia is uncommon and transient during enterocolitis (60).
Arguably the most striking difference between the host responses elicited during typhoid fever and enterocolitis is the type of inflammation observed in the intestine. Typhoid fever and murine typhoid are characterized by a slowly developing infiltrate composed predominantly of mononuclear inflammatory cells, resulting in little or no tissue injury. Mice infected with Salmonella serotype Typhimurium develop a diffuse enteritis in the small intestine within 3 to 5 days postinfection which is characterized by an infiltrate composed predominantly of mononuclear leukocytes (Fig. 1) (87, 90). Biopsies taken from the upper small intestine of volunteers 3 days after experimental infection with Salmonella serotype Typhi or from typhoid fever patients revealed a diffuse enteritis caused predominantly by a mononuclear leukocyte infiltrate (54, 94). The mononuclear cell infiltrate observed during typhoid fever may be associated with localized necrosis of Peyer's patches (usually observed in the second week of infection), but the integrity of the epithelium in other areas of the intestine is largely preserved (6).
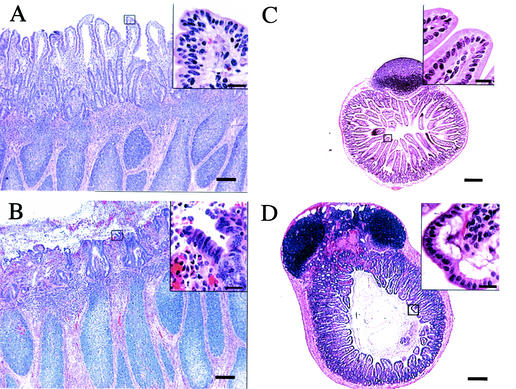
FIG. 1.
Comparative histopathology of the murine and bovine small intestine after infection of calves (A and B) or mice (C and D) with Salmonella serotype Typhimurium strain ATCC 14028. (A) Histological section of an uninfected bovine Peyer's patch (bar = 100 μm). The box covering the tip of an absorptive villus is shown as an enlargement in the insert at the top right (bar = 20 μm). (B) Histopathology of a bovine Peyer's patch at 8 h after infection of a ligated ileal loop with 109 CFU of Salmonella serotype Typhimurium (bar = 100 μm). Note the blunting of villi and the presence of a fibrino-purulent exudate in the intestinal lumen. The box covering the tip of an absorptive villus is shown as an enlargement in the insert at the top right (bar = 20 μm). Note the loss of the integrity of the intestinal epithelium. (C) Histological section of an uninfected murine Peyer's patch (bar = 100 μm). The box covering the tip of an absorptive villus is shown as an enlargement in the insert at the top right (bar = 20 μm). (D) Histopathology of a Peyer's patch from a moribund mouse at 5 days post-oral infection with 109 CFU of Salmonella serotype Typhimurium (bar = 100 μm). The box covering the tip of an absorptive villus is shown as an enlargement in the insert at the top right (bar = 20 μm). Note that the integrity of the intestinal epithelium remains intact.
Enterocolitis, on the other hand, is characterized by a rapidly developing infiltrate which contains neutrophils as the predominant cell type and which is associated with necrosis of the upper mucosa in large areas of the terminal ileum and colon. Rectal biopsies from patients infected with Salmonella serotype Typhimurium reveal an acute enteritis characterized by an inflammatory infiltrate that is composed primarily of neutrophils (17, 65). This influx of neutrophils is associated with necrosis of the uppermost mucosa in large areas of the terminal ileum and colon (61). Collectively, these observations illustrate that Salmonella serotype Typhimurium infections in mice and in humans cause dramatically different intestinal pathologies, elicit different host responses, and result in different disease syndromes. These differences make it difficult to improve our understanding of the pathogenesis of Salmonella serotype Typhimurium infection in humans by extrapolating from data obtained using the mouse model.
Despite the differences in the inflammatory responses observed in the intestines of mice and humans, murine ligated ileal loops have been used as a model to assess the contribution of putative virulence factors to the pathogenesis of Salmonella serotype Typhimurium-induced diarrhea (13). Since the infiltrate elicited by Salmonella serotype Typhimurium in murine ligated ileal loops is composed predominantly of mononuclear cells (2), this model is of questionable relevance for studying the pathogenesis of enterocolitis. In contrast, infection of rabbit ligated ileal loops with Salmonella serotype Typhimurium results in an infiltrate which contains neutrophils as the predominant cell type (19, 32, 33, 104, 106). Since infection of rabbit ligated ileal loops closely mimics the host response encountered during Salmonella serotype Typhimurium infection in humans, this model is used frequently for studying enterocolitis. Oral challenge of rabbits with Salmonella serotype Typhimurium results in a diarrheal disease; however, a shortcoming of this model is that the infection does not remain localized to the intestine and bacteria can typically be isolated from blood, the liver, and the spleen (35). In addition to rabbits, calves have been used to study the pathogenesis of enterocolitis. One Salmonella serotype used to study human enterocolitis in the calf model is serotype Dublin (5, 29, 47, 105). Although Salmonella serotype Dublin infections in young calves commonly manifest as diarrhea, these infections are highly invasive, and meningoencephalitis, polyarthritis, osteomyelitis, or pneumonia may eventually occur in the absence of diarrhea (79). Arguably the most significant shortcoming of using Salmonella serotype Dublin to study human enterocolitis is the fact that this serotype causes bacteremia rather than enterocolitis in humans (20). That is, only about one-third of Salmonella serotype Dublin patients develop diarrhea, while bacteria are cultured from blood in 75 to 91% of cases (20, 97). In contrast, diarrhea is the prominent symptom during human infections with Salmonella serotype Typhimurium and only 1% of human isolates are from blood (98). Furthermore, natural or experimental oral infection in calves with Salmonella serotype Typhimurium results in an enteric disease with clinical and pathological features that parallel the disease in humans (87). Salmonella serotype Typhimurium thus appears to be better suited than Salmonella serotype Dublin to study the pathogenesis of human enterocolitis using the calf model.
Salmonella serotype Typhimurium is the Salmonella serotype most commonly isolated from ill cattle in the United States (80, 111). Upon oral infection with Salmonella serotype Typhimurium, calves develop clinical signs of disease, including diarrhea, anorexia, fever, dehydration, and prostration, within 12 to 48 h (92, 99, 114). Usually, oral inoculations with 104 to 107 CFU cause transient diarrhea that persists for 2 to 8 days, while doses between 108 and 1011 CFU can cause lethal infections (78, 92, 99, 114). Salmonella serotype Typhimurium causes a localized infection in calves, with the most severe pathological lesions being restricted to the intestinal mucosa and mesenteric lymph nodes (99, 114). Animals develop a fibrino-purulent necrotizing enteritis characterized by a severe diffuse infiltrate composed predominantly of neutrophils (99, 114). The neutrophil influx is associated with necrosis of the upper mucosa (Fig. 1), which may result in the formation of a pseudomembrane in the terminal ileum and the cranial 1 to 2 m of the colon (Fig. 2F) (99). The intestinal pathology and the pattern of inflammatory reaction observed in calves parallel those of Salmonella serotype Typhimurium-induced enterocolitis in nonhuman primates (52, 81) and in humans (17, 65). Bovine ligated ileal loops have been used successfully to study fluid accumulation and host responses following Salmonella serotype Typhimurium infection (23, 85, 86). The fact that Salmonella serotype Typhimurium is a natural pathogen of cattle and causes signs of disease and pathology similar to those found in humans infected with this organism makes Salmonella serotype Typhimurium infection of calves an excellent model for studying the pathogenesis of human enterocolitis.
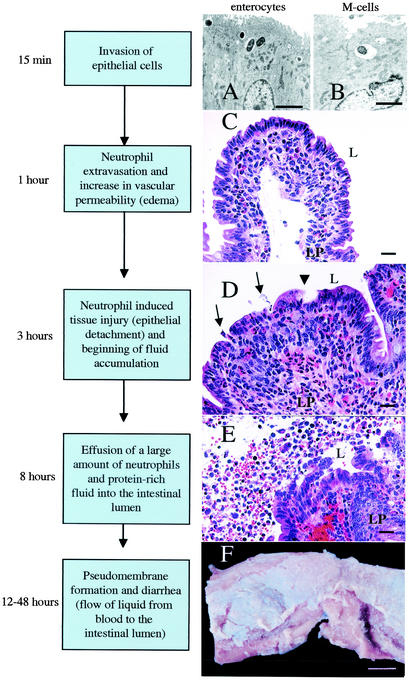
FIG. 2.
Current model of the series of events leading to an inflammatory diarrhea during Salmonella serotype Typhimurium infection of calves. (A) Transmission electron micrograph of bovine Peyer's patch at 15 min after infection of a ligated ileal loop with Salmonella serotype Typhimurium strain ATCC 14028 (109 CFU/loop). Ruffling of the brush border of an enterocyte and bacterial internalization into membrane-bound vacuoles can be seen (bar = 2.5 μm). (B) Transmission electron micrograph of bovine Peyer's patch at 15 min after infection of a ligated ileal loop with Salmonella serotype Typhimurium strain ATCC 14028 (109 CFU/loop). An M cell in the follicle-associated epithelium containing an internalized bacterium is shown (bar = 2.5 μm). (C) Focal infiltration of neutrophils in the lamina propria (LP) of an absorptive villus in bovine Peyer's patches at 1 h after infection of a ligated ileal loop with Salmonella serotype Typhimurium strain ATCC 14028 (109 CFU/loop) (bar = 20 μm). (D) Blunting of absorptive villus 3 h after infection of a ligated ileal loop with Salmonella serotype Typhimurium strain ATCC 14028 (109 CFU/loop). Note the hemorrhage and infiltration of the lamina propria with neutrophils. Arrows indicate areas where neutrophils transmigrate into the intestinal lumen (L). The arrowhead indicates the detachment of surface epithelial cells at the tip of an absorptive villus (bar = 20 μm). (E) Presence of a large number of neutrophils in the intestinal lumen (L) at 8 h postinfection of a ligated ileal loop with Salmonella serotype Typhimurium strain ATCC 14028 (109 CFU/loop). Note the hemorrhage, injury to the intestinal epithelium, and detached enterocytes (bar = 20 μm). (F) Gross pathology of the terminal ileum of a calf at 48 h after oral infection with Salmonella serotype Typhimurium strain ATCC 14028 (1010 CFU). Note the pseudomembrane formation over a bovine Peyer's patch (bar = 1 cm).
PATHOGENESIS OF SALMONELLA SEROTYPE TYPHIMURIUM-INDUCED DIARRHEA: VIRULENCE FACTORS AND CHLORIDE SECRETION
Much of the work on the pathogenesis of enterocolitis is based on the assumption that similar to Vibrio cholerae, Salmonella serotype Typhimurium causes a secretory diarrhea by stimulating chloride secretion. Giannella and coworkers postulated that an increase in cyclic AMP concentration in the mucosa of rabbit ligated ileal loops infected with Salmonella serotype Typhimurium is a mechanism for fluid secretion (34). Giannella also noted that depletion of the neutrophil pool by nitrogen mustard treatment of rabbits markedly reduces Salmonella serotype Typhimurium-induced fluid secretion in ligated ileal loops, thereby suggesting that an inflammatory reaction is important for the pathogenesis of enterocolitis (32). A subsequent study showed that pretreatment of rabbits with nitrogen mustard inhibits fluid secretion in ligated ileal loops induced by both Salmonella serotype Typhimurium and cholera toxin (106). Since cholera toxin, unlike Salmonella serotype Typhimurium, does not cause an inflammatory reaction, it was concluded that the antisecretory effects of nitrogen mustard treatment might not be due to its anti-inflammatory activity but may rather be caused by an inhibition of chloride secretion. These observations initiated a search for a cholera toxin-like activity in Salmonella serotype Typhimurium, and a candidate gene termed stn was finally cloned by hybridization with a DNA probe specific for the cholera toxin genes (ctxAB) (12). Inactivation of the stn gene reduces the ability of Salmonella serotype Typhimurium to induce fluid accumulation in murine ligated ileal loops (13). A lysate of an Escherichia coli strain expressing the Salmonella serotype Typhimurium stn gene causes fluid accumulation in rabbit ligated ileal loops, and this response can be neutralized with anti-cholera toxin antiserum (12, 77). Subsequent sequence analysis revealed that the stn nucleotide sequence and its deduced amino acid sequence have no homology to ctxAB and cholera toxin, respectively (14). Importantly, inactivation of the stn gene does not reduce the ability of Salmonella serotype Typhimurium or Salmonella serotype Dublin to induce fluid accumulation in bovine ligated ileal loops (107, 108, 110).
A number of virulence determinants required for infection of mice have been tested for their role during Salmonella serotype Dublin and Salmonella serotype Typhimurium infection in calves (1, 5, 47, 57, 99-101, 105, 107, 108, 110). Virulence determinants required for growth at systemic sites of infection in mice, including the type III secretion system (TTSS-2) encoded by Salmonella pathogenicity island 2 (SPI-2) and the Salmonella plasmid virulence (spv) genes, contribute to the systemic infection caused by Salmonella serotype Dublin in calves (5, 57, 105). In contrast, SPI-2 and the spv operon are of little or no importance during the localized infection caused by Salmonella serotype Typhimurium in calves (99). These differences likely reflect the fact that Salmonella serotype Typhimurium infection in both calves and humans remains localized to the intestine and mesenteric lymph nodes, while Salmonella serotype Dublin causes a more invasive infection in these two host species (i.e., bacteremia) (20, 87). However, the invasion-associated TTSS-1 encoded by SPI-1 is of equal importance for the intestinal phase of Salmonella serotype Dublin and Salmonella serotype Typhimurium infection in calves. An intact TTSS-1 is essential for eliciting fluid accumulation and neutrophil influx in bovine ligated ileal loops infected with either Salmonella serotype Dublin or Salmonella serotype Typhimurium (1, 107, 108, 110, 115). Furthermore, the TTSS-1 encoded by SPI-1 is required for the ability of Salmonella serotype Typhimurium to cause diarrhea and mortality in calves after oral infection (99, 100, 108, 115). These data suggest that the TTSS-1 is the prime virulence determinant during Salmonella serotype Typhimurium-induced enterocolitis.
The TTSS-1 was initially identified as a virulence factor required for invasion of intestinal epithelial cell lines by Salmonella serotype Typhimurium (28). The main function of the TTSS-1 is to translocate effector proteins into the cytosol of a host cell (26) (Fig. 3). Secreted target proteins are transported across the inner and outer membranes of the bacterial cell by the TTSS-1. A subset of these secreted proteins, including SipB (SspB), SipC (SspC), and SipD (SspD), subsequently form a translocation complex in the eukaryotic membrane that is required for the delivery of other effector proteins into the host cell cytoplasm (15, 24, 29, 37, 113) (Fig. 3). Genes encoding these translocases (SipBCD) and three of the effector proteins, namely SipA (SspA), AvrA, and SptP, are located on SPI-1 (37, 44, 49-51). The remaining TTSS-1 effector proteins, including SopA, SopB (SigD), SopD, SopE1, SopE2, SspH1, and SlrP, are encoded by genes located outside of SPI-1 (Fig. 3) (4, 29, 38, 41, 47, 67, 96, 101, 112, 113). For a detailed discussion of TTSS-1-mediated protein secretion and invasion of host cells the reader is referred to recent review articles (27, 53, 117).
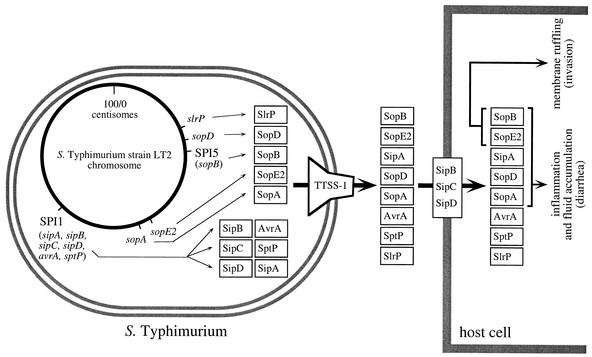
FIG. 3.
Secreted targets of the invasion-associated TTSS-1 of Salmonella serotype Typhimurium and their role in causing diarrhea in calves. The Salmonella serotype Typhimurium chromosome (circle) and the encoded TTSS-1 effector proteins (boxes) are shown in the bacterial cell (left). The TTSS-1 encoded by genes on SPI-1 forms a needle complex spanning the inner and outer membranes of Salmonella serotype Typhimurium (reviewed in reference 53) and is shown on the right side of the bacterial cell. Transport of TTSS-1 effector proteins into the host cell cytosol by the translocation complex formed by SipB, SipC, and SipD is shown on the right. Positions of genes (sopA, sopE2, slrP, and sopD) and pathogenicity islands (SPI-1 and SPI-5) on the physical map of the Salmonella serotype Typhimurium chromosome are based on the complete genome sequence of strain LT2 (63). The TTSS-1 effector genes sspH1 and sopE1 were not included in this figure since they are encoded by bacteriophages that are not present in Salmonella serotype Typhimurium strain LT2.
In Salmonella serotype Dublin, three TTSS-1 effector genes (sopA, sopB, and sopD) have been shown to be required for fluid accumulation and influx of neutrophils in bovine ligated ileal loops (29, 47, 112). SopB is an inositol phosphate phosphatase which alters phosphatidylinositol signaling in the host cell (75, 116). It has been suggested that SopB-mediated changes in phosphatidylinositol signaling may result in chloride secretion by epithelial cells, leading to diarrhea through a secretory mechanism (75, 102, 103). However, the present dogma that Salmonella serotype Typhimurium causes diarrhea by a secretory mechanism is supported only by indirect evidence. The alternate possibility that neutrophil-induced tissue injury is directly responsible for eliciting fluid accumulation by an inflammatory mechanism has never been experimentally refuted.
DOES AN INFLAMMATORY MECHANISM CONTRIBUTE TO SALMONELLA SEROTYPE TYPHIMURIUM-INDUCED DIARRHEA?
Histopathological evaluation of calf intestinal tissue collected between 18 and 48 h after oral infection with Salmonella serotype Typhimurium reveals necrosis of the uppermost mucosa with complete loss of the intestinal epithelium and discernible villi or crypt structures (Fig. 2F) (99). The loss of epithelial cells often affects large surface areas and, in severe cases, covers 3 to 4 m of the terminal ileum and the cranial 1 to 2 m of the colon. The absence of epithelial cells in large areas of the intestine in calves with acute diarrhea raises questions about the importance of chloride secretion by epithelial cells during the pathogenesis of enterocolitis. The intestinal pathology suggests that the increased vascular permeability accompanying inflammation in combination with the loss of epithelial integrity could lead to diarrhea by an exudative mechanism (i.e., flow of water and solutes from the blood to the intestinal lumen as a consequence of inflammation).
Unlike chloride secretion, an inflammatory mechanism of fluid secretion would be expected to result in the release of serum proteins into the intestinal lumen, which is attributable to the loss of the intestinal permeability barrier. A comprehensive evaluation of the hematology and blood chemistry profile of orally infected calves has recently been performed to test the prediction that Salmonella serotype Typhimurium-induced diarrhea results in a nonspecific effusion of serum proteins (83). Calves infected with Salmonella serotype Typhimurium develop neutropenia at days 1 and 2 postinfection, suggestive of severe inflammatory lesions with heavy demand and utilization of neutrophils (83, 92). An increase in the packed cell volume, the hemoglobin concentration, and relative polycytemia suggest that calves become severely dehydrated, with an estimated average decrease in the plasma volume of 15 to 20% (21, 62, 83). Importantly, the concentration of total plasma protein decreases significantly and continuously after infection, indicative of increased intestinal permeability associated with severe intestinal protein loss during Salmonella serotype Typhimurium infection (83, 92). A similar decrease in the concentration of albumin is also detected after infection, indicating that the loss of protein is nonselective (83). These data are consistent with the idea that an inflammatory mechanism contributes significantly to fluid loss during Salmonella serotype Typhimurium-induced enterocolitis.
If Salmonella serotype Typhimurium causes diarrhea mainly by an inflammatory mechanism, then the onset of fluid accumulation in the intestine would be expected to coincide with a loss of the intestinal permeability barrier. This prediction has been tested experimentally using a bovine ligated ileal loop model, in which fluid accumulation and the development of lesions can be examined at defined time points postinfection. Bacterial invasion of epithelial cells (enterocytes and M cells) can be observed within 15 min after infection of bovine ligated ileal loops (Fig. 2A and B). At 1 h postinfection, bacteria can be detected in the lamina propria, where they are localized within mononuclear phagocytes or neutrophils (86). Early inflammatory changes (mild perivascular neutrophil infiltration with a few neutrophils scattered throughout the lamina propria) are present in the mucosa of loops infected with Salmonella serotype Typhimurium at 1 h postinfection (Fig. 2C) and fluid accumulation begins at 3 h postinfection (85). Injury to the intestinal epithelium (i.e., detachment of epithelial cells from the tips of absorptive villi) is first detectable at 3 h postinfection in Salmonella serotype Typhimurium-infected loops, thus coinciding with the onset of fluid accumulation (Fig. 2D) (85, 86). The pathological changes rapidly progress to a severe neutrophilic inflammation associated with necrosis of the mucosa at 8 h (Fig. 1B and 2E), and this is accompanied by an increase in the volume of fluid in the lumen of Salmonella serotype Typhimurium-infected loops (85).
In summary, the experimental evidence currently available is compatible with an inflammatory mechanism for the loss of fluid and protein during Salmonella serotype Typhimurium-induced enterocolitis. It should be pointed out, however, that a contribution of chloride secretion to the severe fluid loss observed in calves infected with Salmonella serotype Typhimurium cannot presently be ruled out. Neutrophils are predicted to play a crucial role in mediating diarrhea by an inflammatory mechanism, since this cell type in particular is known to release substances (e.g., proteases, myeloperoxidase, and reactive oxygen and nitrogen intermediates) that lead to tissue injury. It is also striking that Salmonella serotype Typhimurium induces an inflammatory infiltrate that is overwhelmingly composed of neutrophils and is associated with diarrhea in the bovine and the human host, while the infiltrate in the mouse is dominated by mononuclear cells and is not associated with diarrhea. These data suggest that the study of virulence mechanisms responsible for eliciting this neutrophil-rich inflammatory infiltrate is of prime importance for understanding the pathogenesis of enterocolitis.
TTSS-1 EFFECTOR PROTEINS INVOLVED IN ELICITING AN INFLUX OF NEUTROPHILS AND FLUID ACCUMULATION
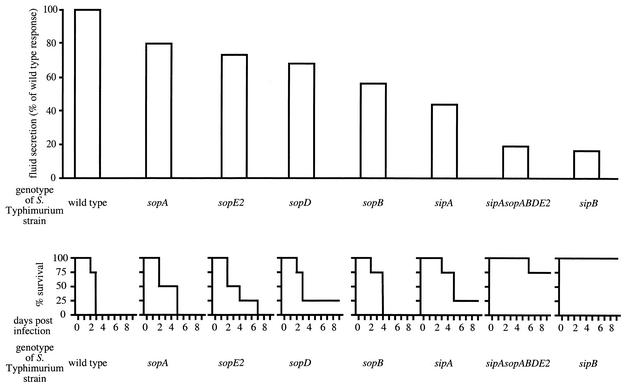
There are two possible mechanisms by which TTSS-1 effector proteins may elicit an inflammatory response in the bovine mucosa. One possibility is that the main role of TTSS-1 effector proteins may be to mediate invasion and transmigration through epithelial cells, which may facilitate recognition by Nods or Toll-like receptors, thereby triggering proinflammatory signaling events (45, 91). In most Salmonella serotype Typhimurium strains, TTSS-1-dependent invasion of epithelial cell lines is mediated by the concerted action of SopB and SopE2 (Fig. 3). A small fraction of Salmonella serotype Typhimurium isolates carry in addition a bacteriophage (SopEΦ) which encodes SopE1, a third TTSS-1 effector protein involved in mediating cytoskeleton rearrangements and bacterial internalization (36, 38, 70). Strains carrying mutations in both sopB and sopE2 (and isolates carrying SopEΦ in addition to a mutation in sopE1) are not able to invade epithelial cell lines (69, 116). In contrast, inactivation of either one of these genes by itself has little effect on invasiveness of Salmonella serotype Typhimurium in vitro (69, 116). Although inactivation of sopB by itself has little effect on Salmonella serotype Typhimurium invasion of epithelial cell lines, it greatly reduces fluid accumulation (Fig. 4) and neutrophil immigration in bovine ligated ileal loops (85). Inactivation of sipA drastically reduces the ability of Salmonella serotype Typhimurium to elicit fluid accumulation (Fig. 4) and an inflammatory response in bovine ligated ileal loops (115) but causes only a short (5 min) delay during invasion of epithelial cell lines in vitro (46, 118). Finally, inactivation of either sopA or sopD has no effect on the ability of Salmonella serotype Typhimurium to enter epithelial cell lines but reduces its ability to elicit fluid accumulation (Fig. 4) and neutrophil immigration in bovine ligated ileal loops (115). The absence of a correlation between the invasiveness of mutants for cultured cell lines and their ability to elicit neutrophil immigration in bovine ligated ileal loops makes it unlikely that the inflammatory response elicited in the bovine mucosa is a mere consequence of bacterial invasion.
FIG. 4.
Role of TTSS-1 effector genes in causing diarrhea in calves. The relative amount of fluid accumulation in bovine ligated ileal loops elicited 8 h after infection with the Salmonella serotype Typhimurium wild type (ATCC 14028) or strains carrying mutations in TTSS-1 effector genes has been reported previously (85, 115) and is shown at the top. Mortality caused in groups of four calves after oral infection at a dose of 1010 CFU/animal with the Salmonella serotype Typhimurium wild type or strains carrying mutations in TTSS-1 effector genes has been reported previously (99, 100, 115) and is shown at the bottom.
An alternative mechanism by which TTSS-1 effector proteins may elicit an inflammatory response is by directly stimulating proinflammatory signaling events in host cells. In vitro studies have shown that the TTSS-1 is directly involved in initiating an inflammatory response by triggering the release of proinflammatory mediators from two different host cell types, macrophages and epithelial cells. Studies with murine macrophage cell lines have found that in addition to its function as a translocase, SipB acts as an effector protein that binds and activates caspase 1, thereby resulting in proteolytic activation of the proinflammatory cytokine interleukin-1β (IL-1β) (39). SipB-dependent cytotoxicity is also observed during infection of bovine macrophages with Salmonella serotype Typhimurium in vitro (84, 109). A second possible mechanism involved in Salmonella serotype Typhimurium-induced inflammation is the TTSS-1-dependent production by intestinal epithelial cells of the chemokine IL-8 (CXCL8/IL-8 by current nomenclature) (40) and of an as yet uncharacterized chemoattractant, known as pathogen-elicited epithelial chemoattractant (64). The TTSS-1-dependent induction of IL-8 in human intestinal epithelial cell lines has been shown to result from an activation of the transcription factors NF-κB and AP-1 (40). A stimulation of nuclear responses and/or the production of proinflammatory mediators is triggered by several TTSS-1 effector proteins in human cell lines in vitro. SopB activates Akt, a serine-threonine kinase that can regulate the transcriptional activity of NF-κB (95). SopE1 and SopE2 activate the mitogen-activated protein kinase JNK through direct interaction with small GTP-binding proteins, including CDC42 and (in the case of SopE1) Rac-1 (22, 36). SipA initiates an ARF6- and PLD-dependent lipid-signaling cascade that elicits the production of an as yet unidentified neutrophil chemoattractant by human intestinal epithelial cell lines (16, 55). Finally, SopA elicits the production of an as yet unidentified neutrophil chemoattractant in human intestinal epithelial cell lines in vitro by an unknown mechanism (112). These data support the idea that TTSS-1 effector proteins elicit the production of proinflammatory mediators in the mucosa by directly engaging targets within host cells. SipB is involved in eliciting the release of proinflammatory mediators from both macrophages (IL-1β) and epithelial cells (IL-8) by acting as an effector protein activating caspase 1 or by acting as a translocase delivering other effector proteins, respectively. However, it is not clear from these in vitro data which mechanisms are important for eliciting neutrophil infiltration in vivo.
Recent work has begun to bridge the gap between in vitro data and studies on the pathogenesis of enterocolitis in vivo. A nonpolar deletion of sipB results in the same degree of attenuation of Salmonella serotype Typhimurium during oral infection of calves as a deletion of genes (prgHIJK) encoding components of the TTSS-1 secretion apparatus (100). These data demonstrate that SipB is essential for enteropathogenicity but do not reveal whether this is attributable to its role as an effector protein or to its function as a translocase. The contribution of SipB-mediated macrophage cell death to diarrheal disease has recently been assessed by in situ detection of terminal deoxyribonucleotidyl transferase-dependent UTP nick end labeling (TUNEL)-positive cells in tissue collected from bovine ligated ileal loops to detect double-strand DNA breaks associated with cell death. The area of TUNEL-positive cells per microscopic field was measured by computer morphometric analysis for both mucosa and lymphoid nodules. This study found that there are no significant differences in the area of TUNEL-positive staining between uninfected controls and loops infected with Salmonella serotype Typhimurium, except at 12 h postinfection, when a significant increase in positive TUNEL staining is detected in infected loops (85). The finding that a significant increase in the number of TUNEL-positive cells is first observed at 12 h post-Salmonella serotype Typhimurium infection, long after the onset of neutrophil influx (1 h postinfection) and tissue injury (3 h postinfection), suggests that the increase in cell death is a consequence rather than a cause of inflammation. Furthermore, these data raise the possibility that attenuation of a sipB mutant is caused by a defect in the translocation of TTSS-1 effector proteins other than SipB itself.
If the main role of SipB is the translocation of effector proteins involved in enteropathogenesis, then inactivation of the genes encoding these effector proteins should reduce the level of inflammation and fluid accumulation in ileal loops to that elicited by a sipB mutant. If, on the other hand, SipB-mediated IL-1β activation in macrophages is required for eliciting inflammation and fluid accumulation, then inactivation of other effector genes would not be expected to reduce the level of inflammation and fluid accumulation to that elicited by a sipB mutant. These alternate predictions have been tested by determining which TTSS-1 effector genes are required for fluid accumulation in bovine ligated ileal loops using mutational analysis (Fig. 4). Salmonella serotype Typhimurium or Salmonella serotype Dublin strains having single mutations in sptP, avrA, sspH1, or slrP induce fluid secretion in the bovine ligated ileal loop model at levels similar to that of an isogenic wild type (89, 115). In contrast, single mutations in sipA, sopA, sopB, sopD, or sopE2 significantly reduce fluid accumulation in bovine ligated ileal loops at 8 h postinfection (29, 47, 85, 112, 115). However, mutations in individual TTSS-1 effector genes, including sipA, sopA, sopB, sopD, and sopE2, do not reduce fluid accumulation as strongly as a mutation in sipB. In contrast, a Salmonella serotype Typhimurium strain carrying mutations in all five TTSS-1 effector genes implicated in eliciting fluid accumulation (i.e., a sipA sopABDE2 mutant) causes the same level of fluid accumulation and inflammation in bovine ligated ileal loops as a strain carrying a mutation in sipB (115). These data suggest that the main role of SipB in eliciting fluid accumulation and inflammation is the translocation of SipA, SopA, SopB, SopD, and SopE2. Furthermore, these data suggest that SipB-mediated activation of IL-1β in macrophages is not essential for eliciting inflammation and fluid accumulation in calves.
In conclusion, SipA, SopA, SopB, SopD, and SopE2 appear to be the main virulence factors responsible for fluid accumulation and inflammation during Salmonella serotype Typhimurium infection of calves. SipA, SopA, SopB, and SopE2 have also been implicated in eliciting the production of proinflammatory mediators by epithelial cells in vitro (16, 22, 55, 95, 112), thereby further supporting the notion that mechanisms responsible for eliciting an inflammatory response are of prime importance for understanding the pathogenesis of Salmonella serotype Typhimurium-induced diarrhea.
CHEMOKINES INVOLVED IN ELICITING THE INFLUX OF NEUTROPHILS
An inflammatory infiltrate overwhelmingly composed of neutrophils is characteristic of Salmonella serotype Typhimurium-induced enterocolitis. The type of inflammatory infiltrate that characterizes a specific disease is controlled, in part, by the subgroup of chemoattractant cytokines expressed in the infected tissue (59). Chemoattractant cytokines are collectively referred to as chemokines and have been divided into two major subfamilies on the basis of the arrangement of the two N-terminal cysteine residues, CXC and CC, depending on whether the first two cysteine residues have an amino acid between them (CXC) or are adjacent to each other (CC) (119). In general, CC chemokines attract predominantly mononuclear leukocytes (59). CXC chemokines can be subdivided further into two groups based on the presence of an N-terminal glutamate-leucine-arginine (ELR) sequence motif preceding the first two cysteines. CXC chemokines containing an ELR motif control the migration of neutrophils, whereas CXC chemokines without the ELR motif attract lymphocytes (3, 59). For instance, viral infections are often accompanied by an inflammatory infiltrate that is dominated by mononuclear leukocytes and is lacking neutrophils, which is thought to be caused by the induction of CC chemokine production with concomitant suppression of CXC chemokine production (8, 93). On the other hand, CXC chemokines containing an ELR motif are produced during diseases characterized by a massive neutrophil influx, such as acute respiratory distress syndrome (11). Hence, current models of chemokine function in directing leukocyte trafficking suggest that the neutrophil influx during Salmonella serotype Typhimurium-induced enterocolitis in humans and cattle is likely due to expression in infected tissue of CXC chemokines containing an ELR motif.
The bovine host is known to express five CXC chemokines containing an ELR motif, including IL-8, granulocyte chemotactic protein 2 (GCP-2, or CXCL6/GCP-2), growth-related gene α (GRO-α, CXCL1/GRO-α, or melanoma growth stimulatory activity [MGSA]), GRO-β (or CXCL2/GRO-β), and GRO-γ (or CXCL3/GROγ) (71-73). The acute severe infiltration of neutrophils observed during infection of bovine ligated ileal loops with Salmonella serotype Typhimurium is associated with increased expression of several CXC chemokines, including IL-8, GCP-2, GRO-α, and GRO-γ, in intestinal tissue (86). Induction of CXC chemokine production has also been observed during infection of human epithelial cell lines with Salmonella serotype Typhimurium in vitro (18, 31, 40, 48). The expression of one of these CXC chemokines, IL-8, is induced in human epithelial cell lines by the Salmonella serotype Typhimurium TTSS-1 in vitro (40). However, the role of TTSS-1 in eliciting expression of GCP-2, GRO-α, GRO-β, or GRO-γ has not yet been investigated. Furthermore, the role of IL-8 during Salmonella serotype Typhimurium-mediated neutrophil recruitment in vivo remains to be determined. Activation of NF-κB and expression of IL-8 by Salmonella serotype Typhimurium-infected intestinal epithelial cells requires an increased cytosolic concentration of calcium (31). Interestingly, down-regulation of a plasma membrane calcium ATPase (PMCA) is observed in bovine Peyer's patches after Salmonella serotype Typhimurium infection (82). Since PMCA pumps calcium into the extracellular environment, decreased expression of PMCA may result in increased levels of cytosolic calcium, thereby favoring IL-8 expression.
The TTSS-1-dependent induction of CXC chemokine expression is likely to be the outcome of a complex interplay involving the interaction between Salmonella serotype Typhimurium and one or more different cell types present in intestinal tissue. To better understand the pathogenesis of enterocolitis it will be necessary to study this complex process in vivo, since the cell types that may participate directly or indirectly in this host pathogen interaction are presently not known. This is not a trivial question, since expression of CXC chemokines such as IL-8 can be induced in vitro upon appropriate stimulation in nearly every type of cell that has been examined (3, 25). Furthermore, in vivo studies will be required to further investigate whether fluid secretion is mediated by an inflammatory mechanism, because increased vascular permeability, edema, and neutrophil influx with subsequent necrosis of the upper mucosa are difficult to reproduce using in vitro models. Future in vivo studies using the calf model are thus expected to be useful and necessary to identify relevant questions that can be investigated further using in vitro models.
Acknowledgments
Work in A.J.B.'s laboratory is supported by Public Health Service grants AI40124 and AI44170. This work was supported in part by USDA/NRICGP no. 2002-35204-11624 to L.G.A. and A.J.B. R.M.T. was supported by USDA/NRICGP no. 9702568. H.A.-P. is presently supported by Public Health Service grant AI052250.
Editor: D. A. Portnoy
REFERENCES
- 1.Ahmer, B. M., J. van Reeuwijk, P. R. Watson, T. S. Wallis, and F. Heffron. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol. Microbiol. 31:971-982. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. W., D. W. Niesel, C. R. Annable, C. B. Hess, M. Asuncion, Y. J. Cho, J. W. Peterson, and G. R. Klimpel. 1993. Tumor necrosis factor-alpha mediates the early pathology in Salmonella infection of the gastrointestinal tract. Microb. Pathog. 14:217-227. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 4.Bakshi, C. S., V. P. Singh, M. W. Wood, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bispham, J., B. N. Tripathi, P. R. Watson, and T. S. Wallis. 2001. Salmonella pathogenicity island 2 influences both systemic salmonellosis and Salmonella-induced enteritis in calves. Infect. Immun. 69:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitar, R., and J. Tarpley. 1985. Intestinal perforation and typhoid fever: a historical and state-of-the-art review. Rev. Infect. Dis. 7:257-271. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., and L. S. Newman. 1982. A review of human salmonellosis. I. Infective dose. Rev. Infect. Dis. 4:1096-1106. [DOI] [PubMed] [Google Scholar]
- 8.Bussfeld, D., M. Nain, P. Hofmann, D. Gemsa, and H. Sprenger. 2000. Selective induction of the monocyte-attracting chemokines MCP-1 and IP-10 in vesicular stomatitis virus-infected human monocytes. J. Interferon Cytokine Res. 20:615-621. [DOI] [PubMed] [Google Scholar]
- 9.Butler, T., W. R. Bell, J. Levin, N. N. Linh, and K. Arnold. 1978. Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch. Intern. Med. 138:407-410. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. 1999. Salmonella surveillance: annual tabulation summary, 1998. U.S. Department of Health and Human Services, CDC, Atlanta, Ga.
- 11.Chollet-Martin, S., P. Montravers, C. Gibert, C. Elbim, J. M. Desmonts, J. Y. Fagon, and M. A. Gougerot-Pocidalo. 1993. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect. Immun. 61:4553-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra, A. K., C. W. Houston, J. W. Peterson, R. Prasad, and J. J. Mekalanos. 1987. Cloning and expression of the Salmonella enterotoxin gene. J. Bacteriol. 169:5095-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra, A. K., J. H. Huang, X. Xu, K. Burden, D. W. Niesel, M. W. Rosenbaum, V. L. Popov, and J. W. Peterson. 1999. Role of Salmonella enterotoxin in overall virulence of the organism. Microb. Pathog. 27:155-171. [DOI] [PubMed] [Google Scholar]
- 14.Chopra, A. K., J. W. Peterson, P. Chary, and R. Prasad. 1994. Molecular characterization of an enterotoxin from Salmonella typhimurium. Microb. Pathog. 16:85-98. [DOI] [PubMed] [Google Scholar]
- 15.Collazo, C. M., and J. E. Galán. 1997. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol. Microbiol. 24:747-756. [DOI] [PubMed] [Google Scholar]
- 16.Criss, A. K., M. Silva, J. E. Casanova, and B. A. McCormick. 2001. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. J. Biol. Chem. 276:48431-48439. [DOI] [PubMed] [Google Scholar]
- 17.Day, D. W., B. K. Mandal, and B. C. Morson. 1978. The rectal biopsy appearances in Salmonella colitis. Histopathology 2:117-131. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 275:14084-14094. [DOI] [PubMed] [Google Scholar]
- 19.Everest, P., J. Ketley, S. Hardy, G. Douce, S. Khan, J. Shea, D. Holden, D. Maskell, and G. Dougan. 1999. Evaluation of Salmonella enterica serovar Typhimurium mutants in a model of experimental gastroenteritis. Infect. Immun. 67:2815-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang, F. C., and J. Fierer. 1991. Human infection with Salmonella dublin. Medicine (Baltimore) 70:198-207. [DOI] [PubMed] [Google Scholar]
- 21.Fisher, E. W., and A. A. Martinez. 1975. Studies of neonatal calf diarrhoea. III. Water balance studies in neonatal salmonellosis. Br. Vet. J. 131:643-652. [DOI] [PubMed] [Google Scholar]
- 22.Friebel, A., H. Ilchmann, M. Aepfelbacher, K. Ehrbar, W. Machleidt, and W. D. Hardt. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035-34040. [DOI] [PubMed] [Google Scholar]
- 23.Frost, A. J., A. P. Bland, and T. S. Wallis. 1997. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet. Pathol. 34:369-386. [DOI] [PubMed] [Google Scholar]
- 24.Fu, Y., and J. E. Galán. 1998. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol. 27:359-368. [DOI] [PubMed] [Google Scholar]
- 25.Furie, M. B., and G. J. Randolph. 1995. Chemokines and tissue injury. Am. J. Pathol. 146:1287-1301. [PMC free article] [PubMed] [Google Scholar]
- 26.Galán, J. E. 1999. Interaction of Salmonella with host cells through the centrisome 63 type III secretion system. Curr. Opin. Microbiol. 2:46-50. [DOI] [PubMed] [Google Scholar]
- 27.Galán, J. E.2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell. Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 28.Galán, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 30.Geoffrey, E., S. Gaines, M. Landy, W. D. Tigertt, H. Sprintz, R.-J. Trapani, A. D. Mandel, and A. S. Benenson. 1960. Studies on infection and immunity in experimental typhoid fever: typhoid fever in chimpanzees orally infected with Salmonella typhosa. J. Exp. Med. 112:143-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gewirtz, A. T., A. S. Rao, P. O. Simon, Jr., D. Merlin, D. Carnes, J. L. Madara, and A. S. Neish. 2000. Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway. J. Clin. Investig. 105:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannella, R. A. 1979. Importance of the intestinal inflammatory reaction in Salmonella-mediated intestinal secretion. Infect. Immun. 23:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannella, R. A., S. B. Formal, G. J. Dammin, and H. Collins. 1973. Pathogenesis of salmonellosis: studies on fluid secretion, and morphologic reaction in the rabbit ileum. J. Clin. Investig. 52:441-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannella, R. A., R. E. Gots, A. N. Charney, W. B. Greenough, and S. B. Formal. 1975. Pathogenesis of Salmonella-mediated intestinal fluid secretion. Gastroenterology 69:1238-1245. [PubMed] [Google Scholar]
- 35.Hanes, D. E., M. G. Robl, C. M. Schneider, and D. H. Burr. 2001. New Zealand White rabbit as a nonsurgical experimental model for Salmonella enterica gastroenteritis. Infect. Immun. 69:6523-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 37.Hardt, W. D., and J. E. Galán. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardt, W. D., H. Urlaub, and J. E. Galán. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. USA 95:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galán. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 41.Hong, K. H., and V. L. Miller. 1998. Identification of a novel Salmonella invasion locus homologous to Shigella flexneri ipgDE. J. Bacteriol. 180:1793-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control. N. Engl. J. Med. 283:686-691. [DOI] [PubMed] [Google Scholar]
- 43.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control 2. N. Engl. J. Med. 283:739-746. [DOI] [PubMed] [Google Scholar]
- 44.Hueck, C. J., M. J. Hantman, V. Bajaj, C. Johnston, C. A. Lee, and S. I. Miller. 1995. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol. Microbiol. 18:479-490. [DOI] [PubMed] [Google Scholar]
- 45.Inohara, N., Y. Ogura, and G. Nunez. 2002. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr. Opin. Microbiol. 5:76-80. [DOI] [PubMed] [Google Scholar]
- 46.Jepson, M. A., B. Kenny, and A. D. Leard. 2001. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell. Microbiol. 3:417-426. [DOI] [PubMed] [Google Scholar]
- 47.Jones, M. A., M. W. Wood, P. B. Mullan, P. R. Watson, T. S. Wallis, and E. E. Galyov. 1998. Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect. Immun. 66:5799-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaniga, K., D. Trollinger, and J. E. Galán. 1995. Identification of two targets of the type III secretion system encoded in inv and spa loci of Salmonella enterica serovar Typhimurium that share homology to IpaD and IpaA proteins. J. Bacteriol. 177:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaniga, K., S. Tucker, D. Trollinger, and J. E. Galán. 1995. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella enterica serovar Typhimurium entry into cultured epithelial cells. J. Bacteriol. 177:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaniga, K., J. Uralil, J. B. Bliska, and J. E. Galán. 1996. A secreted tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21:633-641. [DOI] [PubMed] [Google Scholar]
- 52.Kent, T. H., S. B. Formal, and E. H. Labrec. 1966. Salmonella gastroenteritis in rhesus monkeys. Arch. Pathol. 82:272-279. [PubMed] [Google Scholar]
- 53.Kimbrough, T. G., and S. I. Miller. 2002. Assembly of the type III secretion needle complex of Salmonella typhimurium. Microbes Infect. 4:75-82. [DOI] [PubMed] [Google Scholar]
- 54.Kraus, M. D., B. Amatya, and Y. Kimula. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12:949-955. [PubMed] [Google Scholar]
- 55.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy, E., and W. Gaehtgens. 1908. Über die Verbreitung der Typhusbazillen in den Lymphdrüsen bei Typhusleichen. Arb. Kaiserl. Gesundh. 28:168-171. [Google Scholar]
- 57.Libby, S. J., L. G. Adams, T. A. Ficht, C. Allen, H. A. Whitford, N. A. Buchmeier, S. Bossie, and D. G. Guiney. 1997. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect. Immun. 65:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loeffler, F. 1892. Ueber Epidemieen unter den im hygienishcen Institute zu Greifswald gehaltenen Mäusen und über die Bekämpfung der Feldmausplage. Zentbl. Bakteriol. Parasitenkd. 11:129-141. [Google Scholar]
- 59.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 60.Mandal, B. K., and J. Brennand. 1988. Bacteraemia in salmonellosis: a 15 year retrospective study from a regional infectious diseases unit. BMJ 297:1242-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal, B. K., and V. Mani. 1976. Colonic involvement in salmonellosis. Lancet i:887-888. [DOI] [PubMed] [Google Scholar]
- 62.Mayle, H., A. Seitz, H. Hoerstke, G. Baljer, and G. Dirksen. 1988. The fluid balance in enteral salmonellosis of calves. Dtsch. Tierarztl. Wochenschr. 95:371-374. [PubMed] [Google Scholar]
- 63.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 64.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol. 160:455-466. [PubMed] [Google Scholar]
- 65.McGovern, V. J., and L. J. Slavutin. 1979. Pathology of salmonella colitis. Am. J. Surg. Pathol. 3:483-490. [DOI] [PubMed] [Google Scholar]
- 66.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao, E. A., C. A. Scherer, R. M. Tsolis, R. A. Kingsley, L. G. Adams, A. J. Bäumler, and S. I. Miller. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34:850-864. [DOI] [PubMed] [Google Scholar]
- 68.Miller, S. I., E. L. Hohmann, and D. A. Pegues. 1995. Salmonella (including Salmonella typhi), p. 2013-2033. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed., vol. 2. Churchill Livingstone, New York, N.Y. [Google Scholar]
- 69.Mirold, S., K. Ehrbar, A. Weissmuller, R. Prager, H. Tschäpe, H. Rüssmann, and W. D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1(SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirold, S., W. Rabsch, M. Rohde, S. Stender, H. Tschäpe, H. Rüssmann, E. Igwe, and W. D. Hardt. 1999. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc. Natl. Acad. Sci. USA 96:9845-9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Modi, W. S., M. R. Amarante, M. Hanson, J. E. Womack, and A. Chidambaram. 1998. Assignment of the mouse and cow CXC chemokine genes. Cytogenet. Cell. Genet. 81:213-216. [DOI] [PubMed] [Google Scholar]
- 72.Modi, W. S., and T. Yoshimura. 1999. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol. Biol. E vol. 16:180-193. [DOI] [PubMed] [Google Scholar]
- 73.Morsey, M. A., Y. Popowych, J. Kowalski, G. Gerlach, D. Godson, M. Campos, and L. A. Babiuk. 1996. Molecular cloning and expression of bovine interleukin-8. Microb. Pathog. 20:203-212. [DOI] [PubMed] [Google Scholar]
- 74.Müller, M. 1912. Der Nachweis von Fleischvergiftungsbakterien in Fleisch und Organen von Schlachttieren auf Grund Systematischer Untersuchungen über den Verlauf und den Mechanismus der Infektion des Tierkörpers mit Bakterien der Enteritidis-und Paratyphusgruppe, sowie des Typhus. Zentbl. Bakteriol. Orig. 62:335-373. [Google Scholar]
- 75.Norris, F. A., M. P. Wilson, T. S. Wallis, E. E. Galyov, and P. W. Majerus. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 95:14057-14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76./Orskov, J., and O. Moltke. 1929. Studien über den Infektionsmechanismus bei verschiedenen Paratyphus-Infektionen in weiβen Mäusen. Z. Immunitätsforsch. 59:357-405. [Google Scholar]
- 77.Prasad, R., A. K. Chopra, P. Chary, and J. W. Peterson. 1992. Expression and characterization of the cloned Salmonella typhimurium enterotoxin. Microb. Pathog. 13:109-121. [DOI] [PubMed] [Google Scholar]
- 78.Rankin, J. D., and R. J. Taylor. 1966. The estimation of doses of Salmonella typhimurium suitable for the experimental production of disease in calves. Vet. Rec. 78:706-707. [DOI] [PubMed] [Google Scholar]
- 79.Rings, D. M. 1985. Salmonellosis in calves. Vet. Clin. N. Am. Food Anim. Pract. 1:529-539. [DOI] [PubMed] [Google Scholar]
- 80.Rothenbacher, H. 1965. Mortality and morbidity in calves with salmonellosis. J. Am. Vet. Med. Assoc. 147:1211-1214. [PubMed] [Google Scholar]
- 81.Rout, W. R., S. B. Formal, G. J. Dammin, and R. A. Giannella. 1974. Pathophysiology of Salmonella diarrhea in the rhesus monkey: intestinal transport, morphological and bacteriological studies. Gastroenterology 67:59-70. [PubMed] [Google Scholar]
- 82.Santos, R. L., J. A. Schoffelmeer, R. M. Tsolis, J. A. G. Gutiérrez-Pabello, A. J. Bäumler, and L. G. Adams. 2002. Salmonella typhimurium infection of bovine Peyer's patches downregulates plasma membrane calcium transporting ATPase expression. J. Infect. Dis. 186: 372-378. [DOI] [PubMed] [Google Scholar]
- 83.Santos, R. L., R. M. Tsolis, A. J. Bäumler, and L. G. Adams. 2002. Dynamics of hematologic and blood chemical changes in Salmonella typhimurium infected calves. Am. J. Vet. Res. 63:1145-1150. [DOI] [PubMed] [Google Scholar]
- 84.Santos, R. L., R. M. Tsolis, A. J. Bäumler, R. Smith, 3rd, and L. G. Adams. 2001. Salmonella enterica serovar Typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms. Infect. Immun. 69:2293-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos, R. L., R. M. Tsolis, S. Zhang, T. A. Ficht, A. J. Bäumler, and L. G. Adams. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 69:4610-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santos, R. L., S. Zhang, R. M. Tsolis, A. J. Bäumler, and L. G. Adams. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39:200-215. [DOI] [PubMed] [Google Scholar]
- 87.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Bäumler. 2002. Animal models of Salmonella infections: enteritis vs. typhoid fever. Microb. Infect. 3:237-247. [DOI] [PubMed] [Google Scholar]
- 88.Saphra, I., and M. Wassermann. 1954. Salmonella cholerae suis. A clinical and epidemiological evaluation of 329 infections identified between 1940 and 1954 in the New York Salmonella Center. Am. J. Med. Sci. 228:525-533. [DOI] [PubMed] [Google Scholar]
- 89.Schesser, K., J. M. Dukuzumuremyi, C. Cilio, S. Borg, T. S. Wallis, S. Pettersson, and E. E. Galyov. 2000. The salmonella YopJ-homologue AvrA does not possess YopJ-like activity. Microb. Pathog. 28:59-70. [DOI] [PubMed] [Google Scholar]
- 90.Shirai, Y., K. Sunakawa, Y. Ichihashi, and H. Yamaguchi. 1979. A morphological study in germfree mice (Salmonella infection). Exp. Pathol. 17:158-166. [DOI] [PubMed] [Google Scholar]
- 91.Sieling, P. A., and R. L. Modlin. 2002. Toll-like receptors: mammalian “taste receptors” for a smorgasbord of microbial invaders. Curr. Opin. Microbiol. 5:70-75. [DOI] [PubMed] [Google Scholar]
- 92.Smith, B. P., F. Habasha, M. Reina-Guerra, and A. J. Hardy. 1979. Bovine salmonellosis: experimental production and characterization of the disease in calves, using oral challenge with Salmonella typhimurium. Am. J. Vet. Res. 40:1510-1513. [PubMed] [Google Scholar]
- 93.Sprenger, H., R. G. Meyer, A. Kaufmann, D. Bussfeld, E. Rischkowsky, and D. Gemsa. 1996. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J. Exp. Med. 184:1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sprinz, H., E. J. Gangarosa, M. Williams, R. B. Hornick, and T. E. Woodward. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am. J. Dig. Dis. 11:615-624. [DOI] [PubMed] [Google Scholar]
- 95.Steele-Mortimer, O., L. A. Knodler, S. L. Marcus, M. P. Scheid, B. Goh, C. G. Pfeifer, V. Duronio, and B. B. Finlay. 2000. Activation of Akt/protein kinase B in epithelial cells by the salmonella typhimurium effector sigD. J. Biol. Chem. 275:37718-37724. [DOI] [PubMed] [Google Scholar]
- 96.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 97.Taylor, D. N., J. M. Bied, J. S. Munro, and R. A. Feldman. 1982. Salmonella dublin infections in the United States, 1979-1980. J. Infect. Dis. 146: 322-327. [DOI] [PubMed] [Google Scholar]
- 98.Threlfall, E. J., M. L. Hall, and B. Rowe. 1992. Salmonella bacteraemia in England and Wales, 1981-1990. J. Clin. Pathol. 45:34-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Bäumler. 1999. Contribution of Salmonella enterica serovar Typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsolis, R. M., L. G. Adams, M. J. Hantman, C. A. Scherer, T. Kimborough, R. A. Kingsley, T. A. Ficht, S. I. Miller, and A. J. Bäumler. 2000. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsolis, R. M., S. M. Townsend, E. A. Miao, S. I. Miller, T. A. Ficht, L. G. Adams, and A. J. Bäumler. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uzzau, S., and A. Fasano. 2000. Cross-talk between enteric pathogens and the intestine. Cell. Microbiol. 2:83-89. [DOI] [PubMed] [Google Scholar]
- 103.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]
- 104.Wallis, T. S., R. J. Hawker, D. C. Candy, G. M. Qi, G. J. Clarke, K. J. Worton, M. P. Osborne, and J. Stephen. 1989. Quantification of the leucocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. J. Med. Microbiol. 30:149-156. [DOI] [PubMed] [Google Scholar]
- 105.Wallis, T. S., S. M. Paulin, J. S. Plested, P. R. Watson, and P. W. Jones. 1995. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect. Immun. 63:2755-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wallis, T. S., A. T. Vaughan, G. J. Clarke, G. M. Qi, K. J. Worton, D. C. Candy, M. P. Osborne, and J. Stephen. 1990. The role of leucocytes in the induction of fluid secretion by Salmonella typhimurium. J. Med. Microbiol. 31:27-35. [DOI] [PubMed] [Google Scholar]
- 107.Wallis, T. S., M. Wood, P. Watson, S. Paulin, M. Jones, and E. Galyov. 1999. Sips, Sops, and SPIs but not Stn influence Salmonella enteropathogenesis. Adv. Exp. Med. Biol. 473:275-280. [DOI] [PubMed] [Google Scholar]
- 108.Watson, P. R., E. E. Galyov, S. M. Paulin, P. W. Jones, and T. S. Wallis. 1998. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66:1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watson, P. R., A. V. Gautier, S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis. 2000. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect. Immun. 68:3744-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watson, P. R., S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis. 1995. Characterization of intestinal invasion by Salmonella enterica serovar Typhimurium and Salmonella dublin and effect of a mutation in the invH gene. Infect. Immun. 63:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wells, S. J., S. L. Ott, and A. H. Seitzinger. 1998. Key health issues for dairy cattle—new and old. J. Dairy Sci. 81:3029-3035. [DOI] [PubMed] [Google Scholar]
- 112.Wood, M. W., M. A. Jones, P. R. Watson, A. M. Siber, B. A. McCormick, S. Hedges, R. Rosqvist, T. S. Wallis, and E. E. Galyov. 2000. The secreted effector protein of Salmonella dublin, SopA, is translocated into eukaryotic cells and influences the induction of enteritis. Cell. Microbiol. 2:293-303. [DOI] [PubMed] [Google Scholar]
- 113.Wood, M. W., R. Rosqvist, P. B. Mullan, M. H. Edwards, and E. E. Galyov. 1996. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327-338. [DOI] [PubMed] [Google Scholar]
- 114.Wray, C., and W. J. Sojka. 1978. Experimental Salmonella typhimurium infection in calves. Res. Vet. Sci. 25: 139-143. [PubMed] [Google Scholar]
- 115.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. SipA, SopA, SopB, SopD and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galán. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]
- 117.Zhou, D., and J. Galán. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293-1298. [DOI] [PubMed] [Google Scholar]
- 118.Zhou, D., M. S. Mooseker, and J. E. Galán. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]
- 119.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]