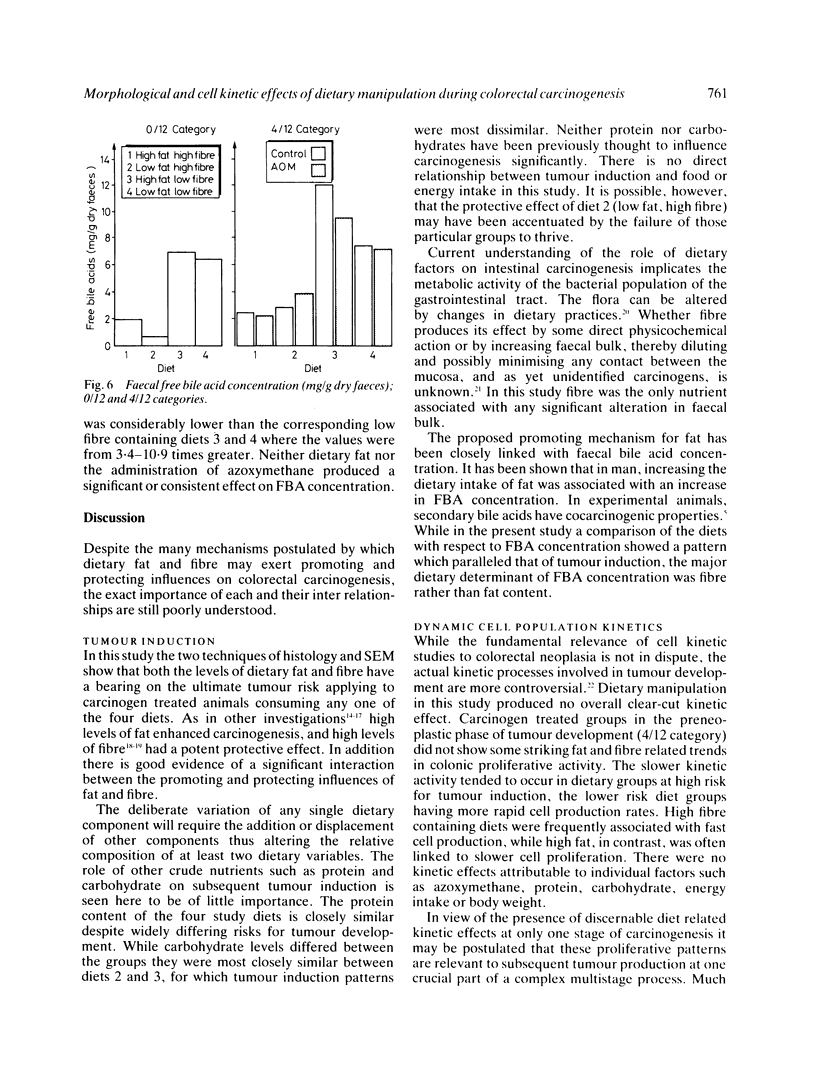
Abstract
The effect of dietary manipulation of fat and fibre on the structural and cell kinetic characteristics of colonic mucosa was studied before and during experimental carcinogenesis in 232 male Albino Swiss rats. Carcinogen treated animals were given 12 weekly injections of azoxymethane (10 mg/kg/week). The animals were divided between four dietary groups (1) high fat, high fibre, (2) low fat, high fibre, (3) high fat, low fibre and (4) low fat, low fibre. Pathological and cell kinetic information together with details of certain faecal characteristics was collected when the animals were killed 4, 20, and 28 weeks after starting their experimental diet. Tumour induction was significantly influenced by diet. The highest risk of colorectal tumour development was found in groups fed diet 3: high fat, low fibre (p less than 0.03). In contrast, diet 2: low fat, high fibre was associated with the lowest risk. The proportion of histologically proven colonic tumours occurring in each dietary group was: diet 1-10.9%, diet 2-3.6%, diet 3-63.7%, diet 4-21.8%. Scanning electron microscopic (SEM) studies done on selected samples indicated both dietary and azoxymethane related alterations in crypt unit integrity. The most marked surface architectural changes were seen in carcinogen treated animals maintained on diet 3 (high fat, low fibre). Stathmokinetic analysis revealed considerable intergroup variability. Both fat and fibre produced significant effects, principally during the preneoplastic phase of carcinogenesis. Faster proliferative activity tended to be found in animals at low risk of tumour induction (diet 2), slower proliferation being more characteristic of animals at high risk (p less than 0.05). The findings suggest that both topographical and cell kinetic parameters have an important relationship with promoting and protecting dietary factors during the development of colorectal cancer.
Full text
PDF

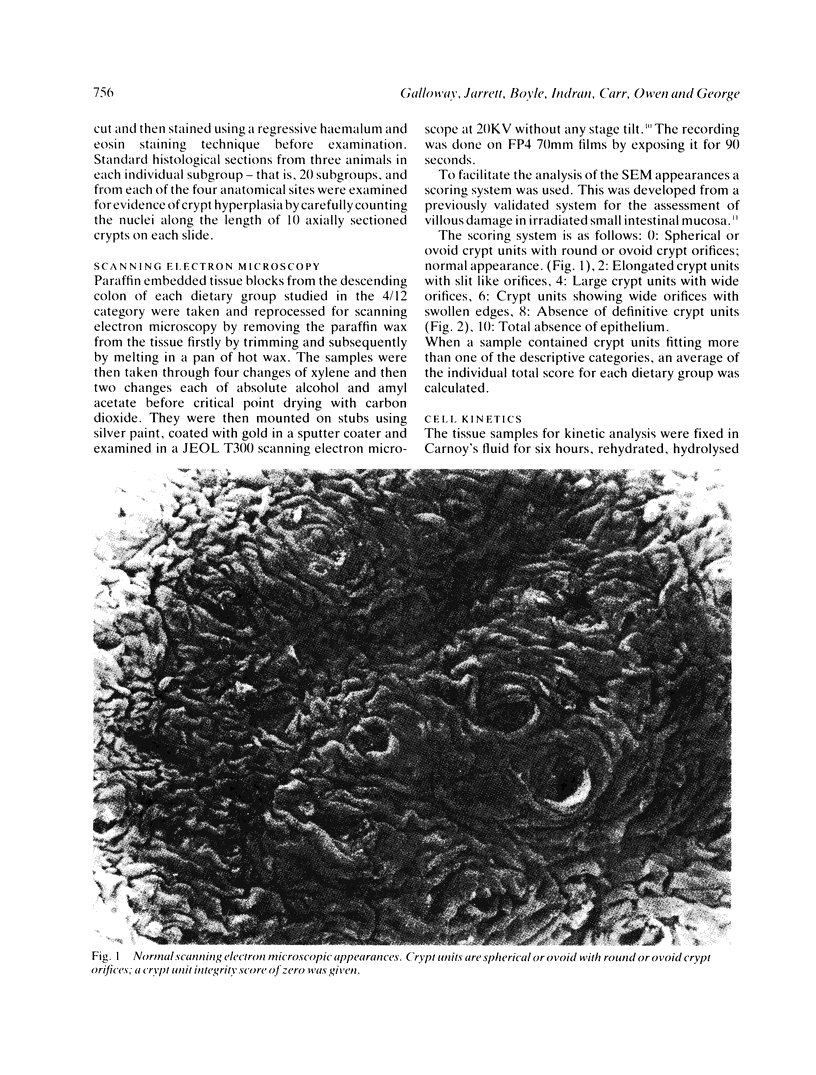
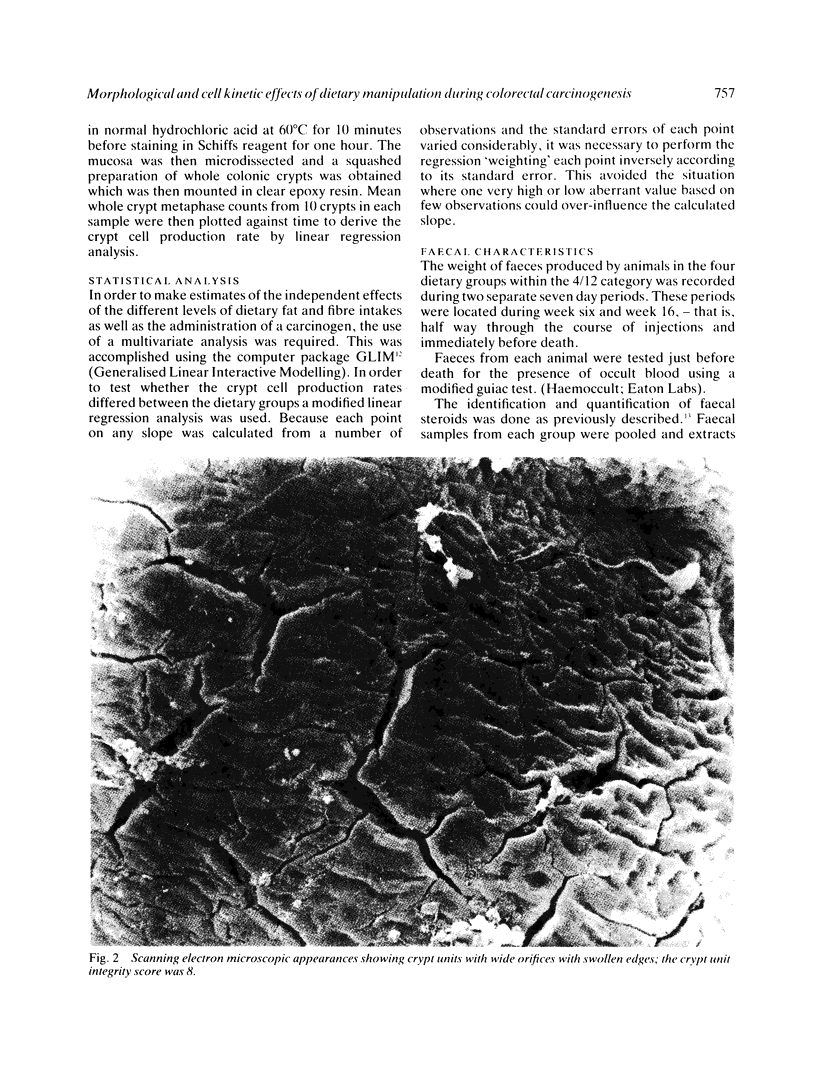

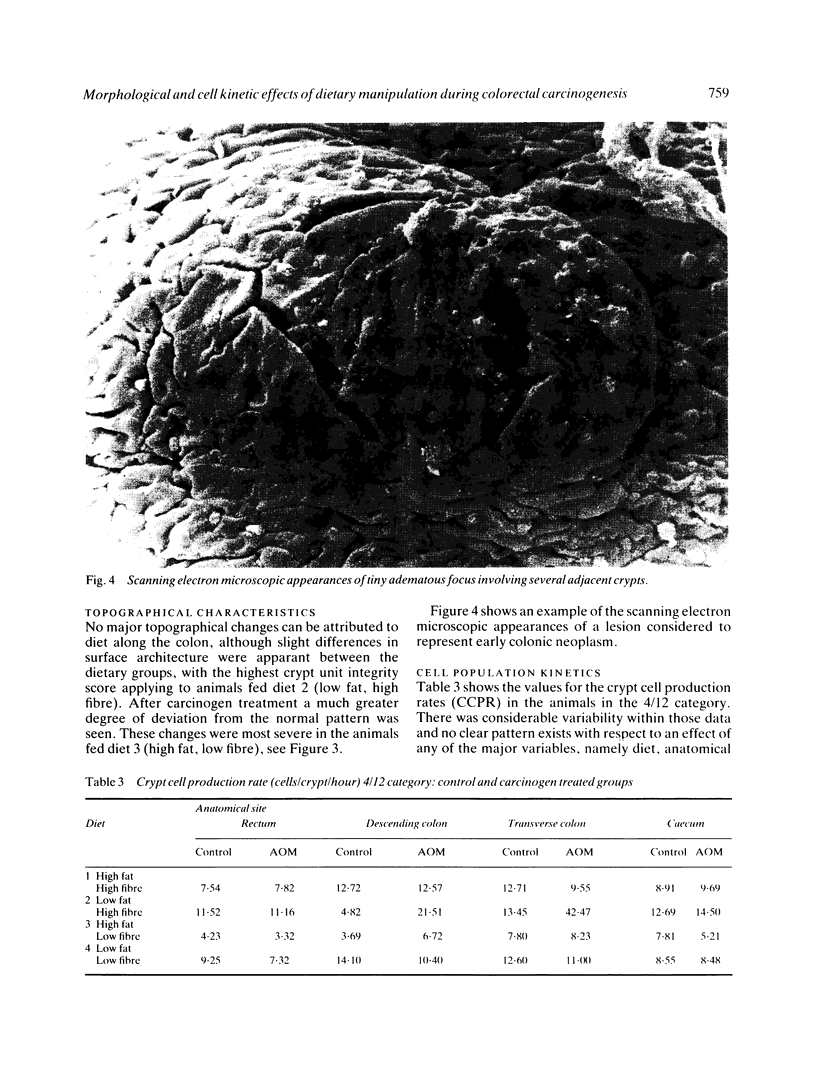
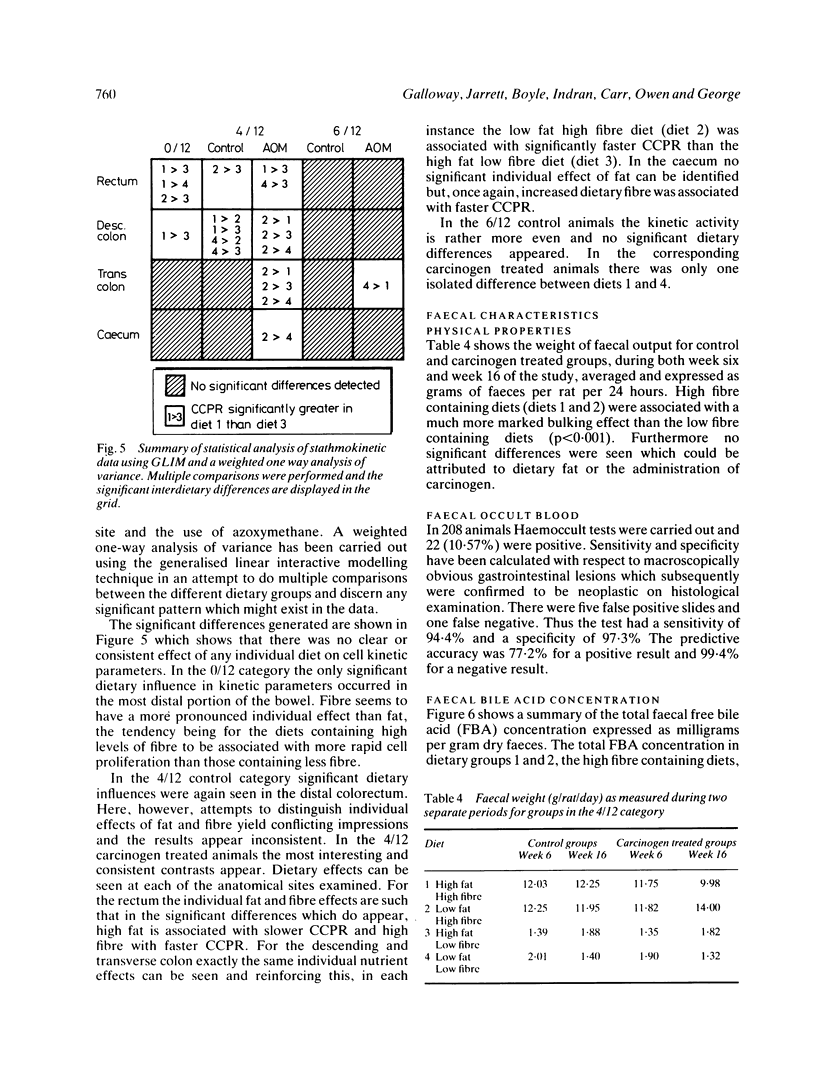


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Crowther J. S., Drasar B. S., Hill M. J., Williams R. E. Bacteria and the aetiology of cancer of the large bowel. Gut. 1969 May;10(5):334–335. doi: 10.1136/gut.10.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. Modification of early dimethylhydrazine carcinogenesis by colonic mucosal hyperplasia. Cancer Res. 1980 Dec;40(12):4451–4455. [PubMed] [Google Scholar]
- Broitman S. A., Vitale J. J., Vavrousek-Jakuba E., Gottlieb L. S. Polyunsaturated fat, cholesterol and large bowel tumorigenesis. Cancer. 1977 Nov;40(5 Suppl):2455–2463. doi: 10.1002/1097-0142(197711)40:5+<2455::aid-cncr2820400911>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Carr K. E., Hamlet R., Nias A. H., Watt C. Damage to the surface of the small intestinal villus: an objective scale of assessment of the effects of single and fractionated radiation doses. Br J Radiol. 1983 Jul;56(667):467–475. doi: 10.1259/0007-1285-56-667-467. [DOI] [PubMed] [Google Scholar]
- Carr K. E., McLay A. L., Toner P. G., Chung P., Wong A. SEM in service pathology: a review of its potential role. Scan Electron Microsc. 1980;(3):121–138. [PubMed] [Google Scholar]
- Cooke T., Kirkham N., Stainthorp D. H., Inman C., Goeting N., Taylor I. Detection of early neoplastic changes in experimentally induced colorectal cancer using scanning electron microscopy and cell kinetic studies. Gut. 1984 Jul;25(7):748–755. doi: 10.1136/gut.25.7.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P., Haenszel W. The epidemiology of large-bowel cancer. Adv Cancer Res. 1978;26:1–141. doi: 10.1016/s0065-230x(08)60086-x. [DOI] [PubMed] [Google Scholar]
- Fleiszer D. M., Murray D., Richards G. K., Brown R. A. Effects of diet on chemically induced bowel cancer. Can J Surg. 1980 Jan;23(1):67–73. [PubMed] [Google Scholar]
- Freeman H. J., Spiller G. A., Kim Y. S. A double-blind study on the effect of purified cellulose dietary fiber on 1,2-dimethylhydrazine-induced rat colonic neoplasia. Cancer Res. 1978 Sep;38(9):2912–2917. [PubMed] [Google Scholar]
- Hill M. J. The effect of some factors on the faecal concentration of acid steroids, neutral steroids and urobilins. J Pathol. 1971 Aug;104(4):239–245. doi: 10.1002/path.1711040405. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R. Enhancement of rat colon carcinogenesis by wheat bran consumption during the stage of 1,2-dimethylhydrazine administration. Cancer Res. 1983 Sep;43(9):4057–4061. [PubMed] [Google Scholar]
- Nigro N. D., Singh D. V., Campbell R. L., Sook M. Effect of dietary beef fat on intestinal tumor formation by azoxymethane in rats. J Natl Cancer Inst. 1975 Feb;54(2):439–442. [PubMed] [Google Scholar]
- Owen R. W., Thompson M. H., Hill M. J. Analysis of metabolic profiles of steroids in faeces of healthy subjects undergoing chenodeoxycholic acid treatment by liquid-gel chromatography and gas-liquid chromatography-mass spectrometry. J Steroid Biochem. 1984 Nov;21(5):593–600. doi: 10.1016/0022-4731(84)90336-4. [DOI] [PubMed] [Google Scholar]
- Pozharisski K. M. The significance of nonspecific injury for colon carcinogenesis in rats. Cancer Res. 1975 Dec;35(12):3824–3830. [PubMed] [Google Scholar]
- Reddy B. S., Narisawa T., Vukusich D., Weisburger J. H., Wynder E. L. Effect of quality and quantity of dietary fat and dimethylhydrazine in colon carcinogenesis in rats. Proc Soc Exp Biol Med. 1976 Feb;151(2):237–239. doi: 10.3181/00379727-151-39181. [DOI] [PubMed] [Google Scholar]
- Trudel J. L., Senterman M. K., Brown R. A. The fat/fiber antagonism in experimental colon carcinogenesis. Surgery. 1983 Oct;94(4):691–696. [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Cell proliferation in the descending colon of dimethylhydrazine treated rats and in dimethylhydrazine induced adenocarcinomata. Virchows Arch B Cell Pathol. 1976 Aug 11;21(2):147–160. doi: 10.1007/BF02899151. [DOI] [PubMed] [Google Scholar]
- Williamson R. C., Davies P. W., Bristol J. B., Wells M. Intestinal adaptation and experimental carcinogenesis after partial colectomy. Increased tumour yields are confined to the anastomosis. Gut. 1982 Apr;23(4):316–325. doi: 10.1136/gut.23.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Appleton D. R. The metaphase arrest technique. A critical review. Cell Tissue Kinet. 1980 Nov;13(6):643–663. doi: 10.1111/j.1365-2184.1980.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Wynder E. L., Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967 Sep;20(9):1520–1561. doi: 10.1002/1097-0142(196709)20:9<1520::aid-cncr2820200920>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]





