Abstract
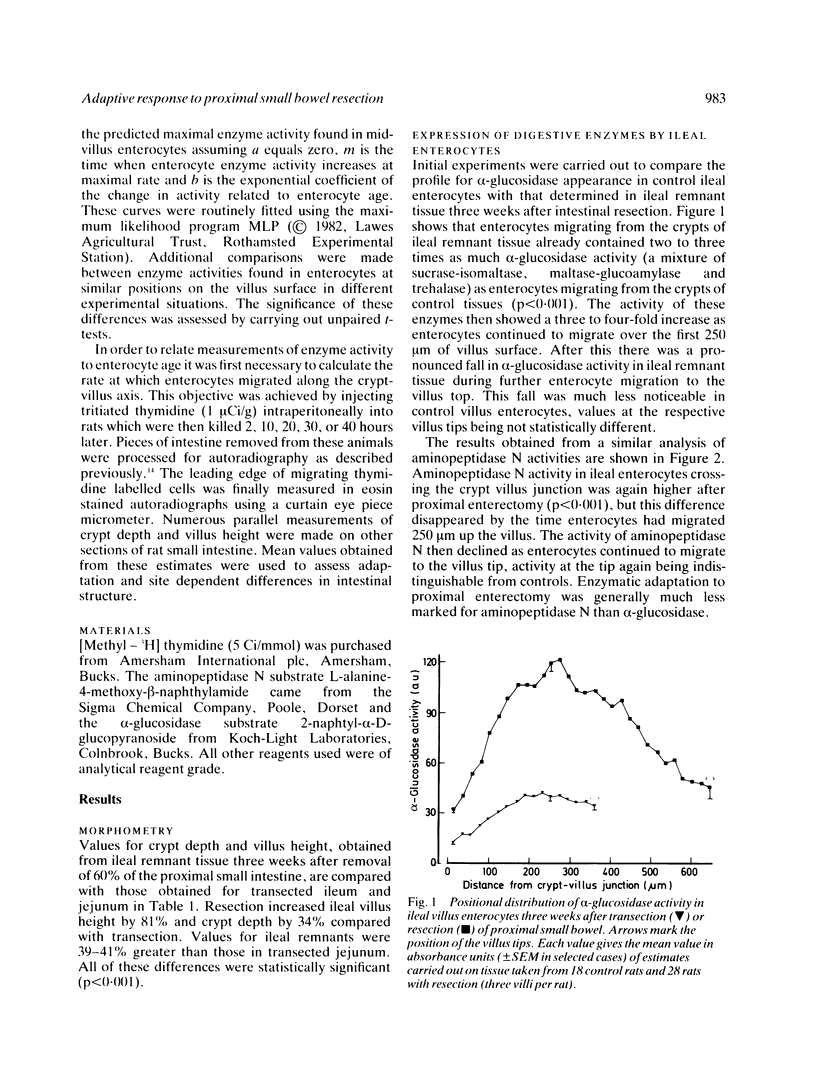
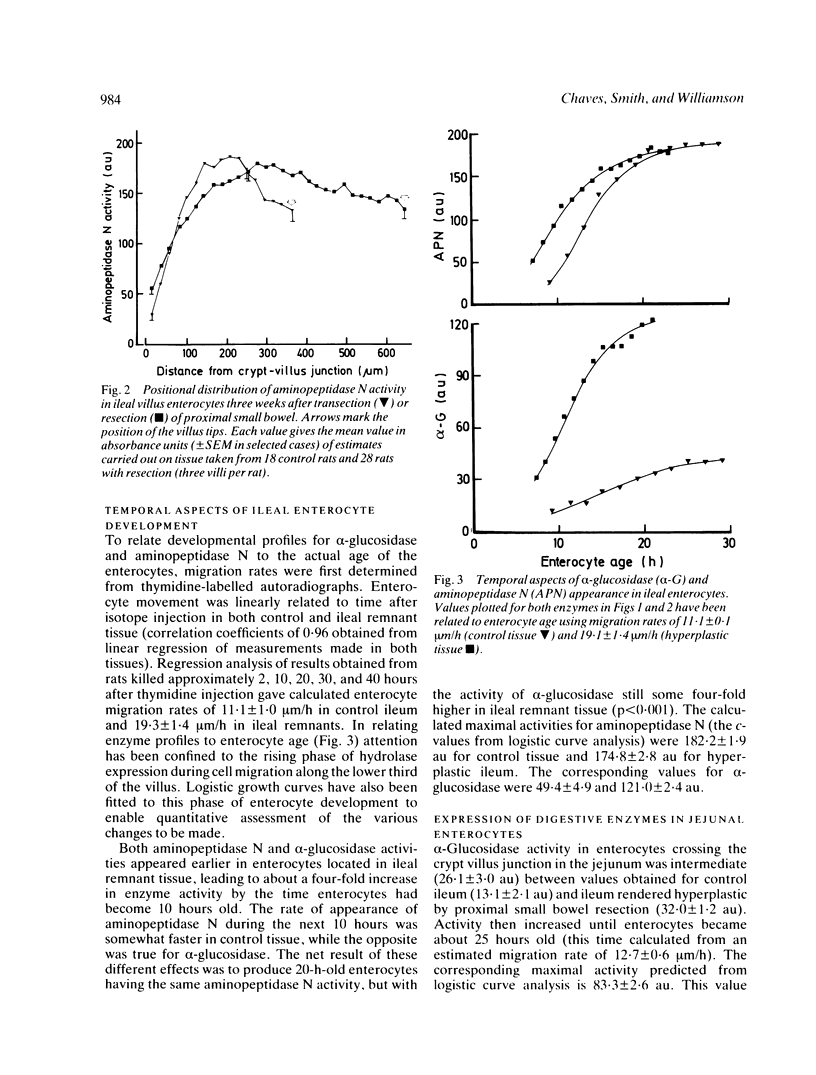
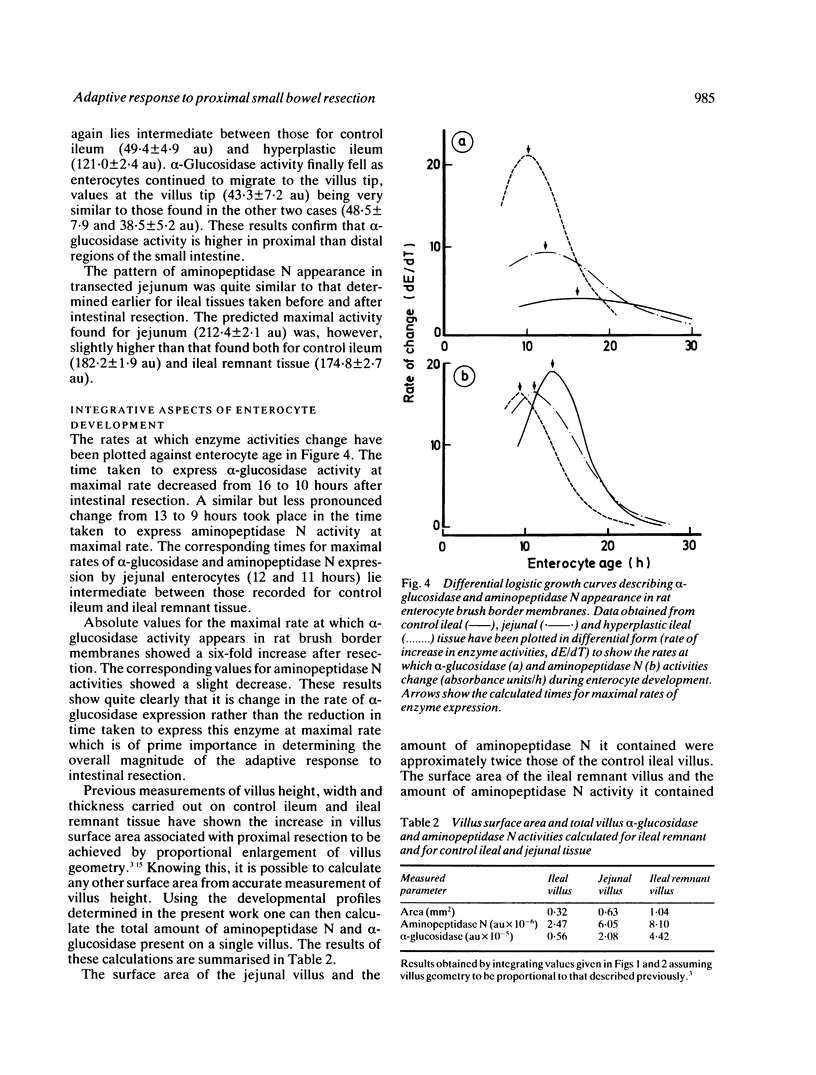
The ability of adapting ileal enterocytes to express different digestive enzymes in their brush border membranes was tested in young female Wistar rats (n = 72) receiving 60% proximal small bowel resection. In control rats with intestinal transection both neutral aminopeptidase and alpha-glucosidase activities were shown, by quantitative cytochemistry, to increase during enterocyte migration over the lower part of the villus; thereafter enzyme activities declined or remained approximately constant. Proximal enterectomy increased the amount of alpha-glucosidase but not neutral aminopeptidase activity appearing during early enterocyte development. Thymidine labelled autoradiography showed that the rate of enterocyte migration along the ileal villus nearly doubled after jejunal resection (19.3 v 11.1 microns/h). Nevertheless, the time taken for both peptidase and saccharidase activities to appear at maximal rates in the brush border membrane was diminished by about five hours. Thus ileal enterocytes adapt to proximal small bowel resection by selective increments in enzyme expression, findings that contradict the previous hypothesis of simple metabolic immaturity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bury K. D. Carbohydrate digestion and absorption after massive resection of the small intestine. Surg Gynecol Obstet. 1972 Aug;135(2):177–187. [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet. 1966 Jul 16;2(7455):146–147. doi: 10.1016/s0140-6736(66)92426-3. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dowling R. H. Small bowel adaptation and its regulation. Scand J Gastroenterol Suppl. 1982;74:53–74. [PubMed] [Google Scholar]
- Fehlmann M., Starita-Geribaldi M., Thiebaut C., Sudaka P. Effect of massive proximal small bowel resection on intestinal brush border membrane proteins in the dog. Arch Int Physiol Biochim. 1978 Aug;86(3):601–612. doi: 10.3109/13813457809055928. [DOI] [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Menge H., Riecken E. O. The adaptive response of disaccharidase activities at different sites along the villus epithelium after proximal intestinal resection in the rat. A microdensitometric study of enzyme kinetics. Res Exp Med (Berl) 1983;182(3):203–213. doi: 10.1007/BF01851709. [DOI] [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Riecken E. O. A quantitative histochemical technique for the characterisation of alpha-glucosidases in the brush-border membrane of rat jejunum. Histochemistry. 1979 Sep;63(1):81–101. doi: 10.1007/BF00508014. [DOI] [PubMed] [Google Scholar]
- King I. S., Paterson J. Y., Peacock M. A., Smith M. W., Syme G. Effect of diet upon enterocyte differentiation in the rat jejunum. J Physiol. 1983 Nov;344:465–481. doi: 10.1113/jphysiol.1983.sp014952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M., Kim Y. S. Changes in sucrase, enterokinase, and peptide hydrolase after intestinal resection. The association of cellular hyperplasia and adaptation. J Clin Invest. 1973 Apr;52(4):942–951. doi: 10.1172/JCI107259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge H., Hopert R., Alexopoulos T., Riecken E. O. Three-dimensional structure and cell kinetics at different sites of rat intestinal remnants during the early adaptive response to resection. Res Exp Med (Berl) 1982;181(2):77–94. doi: 10.1007/BF01852185. [DOI] [PubMed] [Google Scholar]
- Menge H., Robinson J. W. The relationship between the functional and structural alterations in the rat small intestine following proximal resection of varying extents. Res Exp Med (Berl) 1978 Jul 24;173(1):41–53. doi: 10.1007/BF01851373. [DOI] [PubMed] [Google Scholar]
- Menge H., Sepúlveda F. V., Smith M. W. Cellular adaptation of amino acid transport following intestinal resection in the rat. J Physiol. 1983 Jan;334:213–223. doi: 10.1113/jphysiol.1983.sp014490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., CRAWFORD D. T., SELIGMAN A. M. The histochemical demonstration of leucine aminopeptidase. J Histochem Cytochem. 1957 May;5(3):264–278. doi: 10.1177/5.3.264. [DOI] [PubMed] [Google Scholar]
- Robinson J. W., Van Melle G., Riecken E. O., Menge H. Structural and functional correlations in the hypertrophic mucosa of intestinal remnants following resection in rats. Res Exp Med (Berl) 1982;181(2):95–104. doi: 10.1007/BF01852186. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Expression of digestive and absorptive function in differentiating enterocytes. Annu Rev Physiol. 1985;47:247–260. doi: 10.1146/annurev.ph.47.030185.001335. [DOI] [PubMed] [Google Scholar]
- Triadou N., Bataille J., Schmitz J. Longitudinal study of the human intestinal brush border membrane proteins. Distribution of the main disaccharidases and peptidases. Gastroenterology. 1983 Dec;85(6):1326–1332. [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]


