Abstract
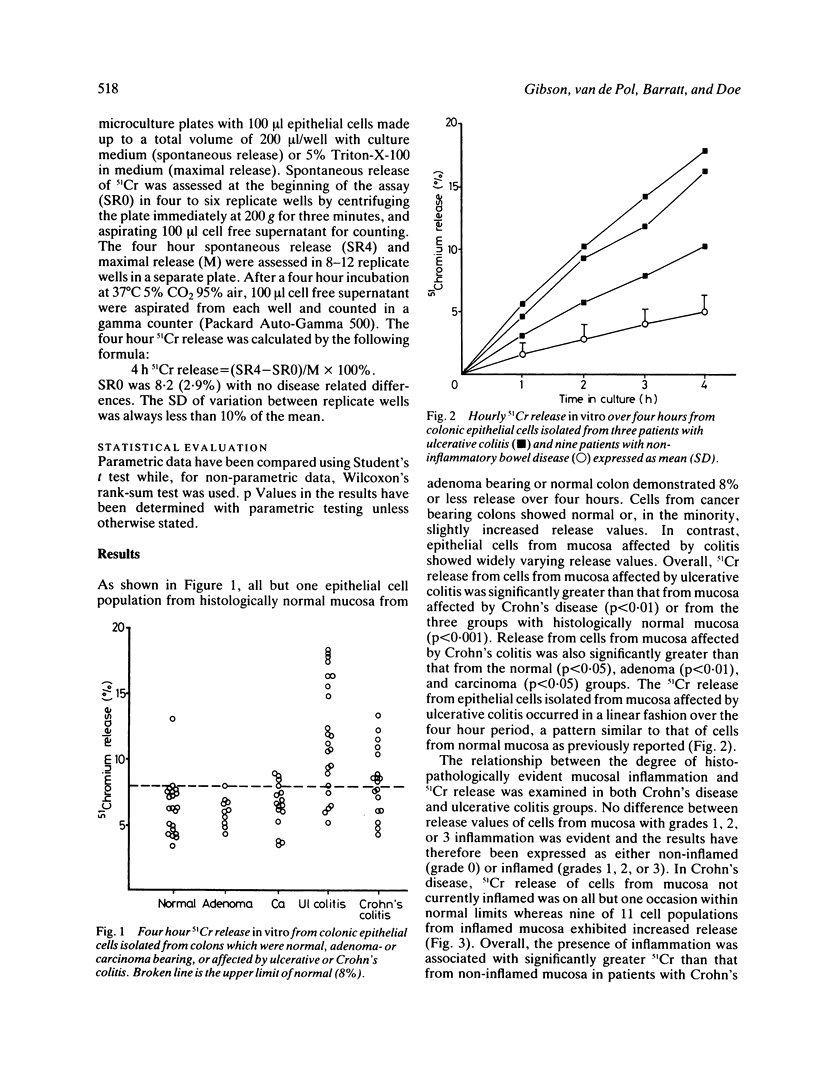
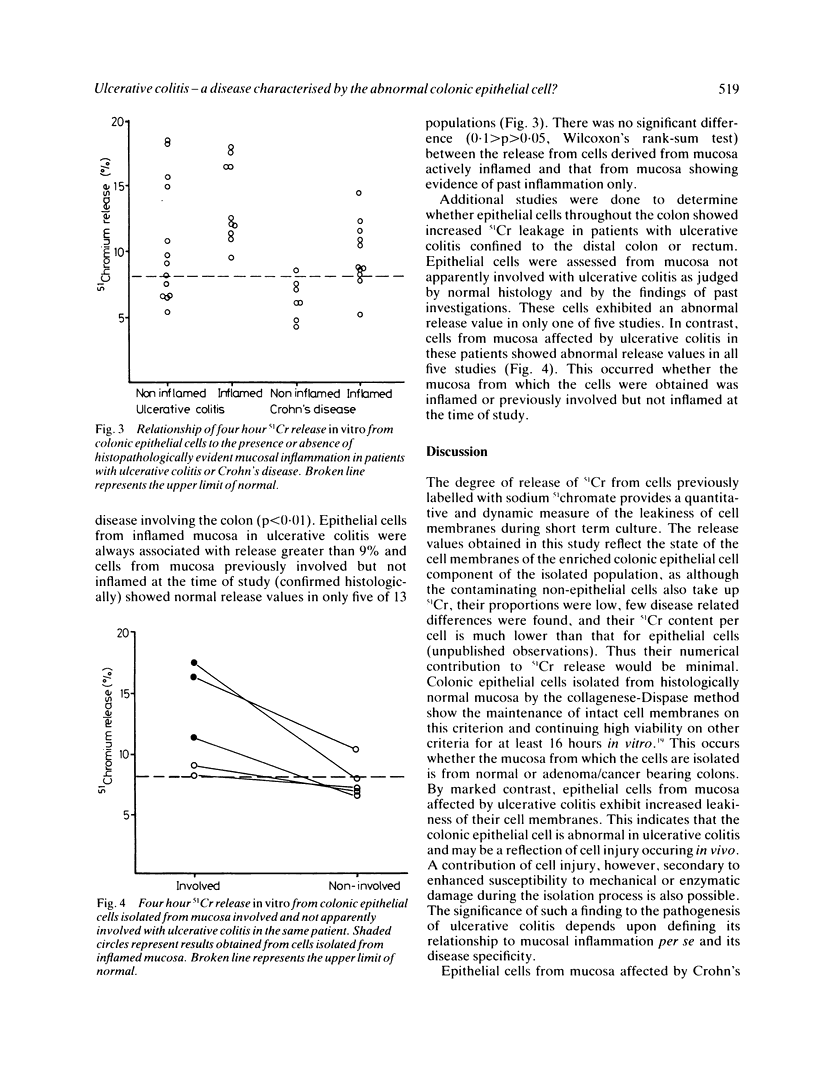
The leakiness of the cell membranes of colonic epithelial cells isolated by the collagenase/Dispase technique from normal or diseased colons was assessed in a 4 h 51Cr release assay. Cells from normal, adenoma bearing or cancer bearing colons showed 51Cr release of 8% or less in almost all of 46 cell populations tested. In contrast, cells from mucosa affected by ulcerative colitis [11.9 (4.3%) n = 23] or Crohn's disease [8.4 (2.7%) n = 18] released significantly more 51Cr than the non-inflamed groups. Values are expressed as mean (SD). Overall, release values were greater in ulcerative colitis than Crohn's disease (p less than 0.01). In Crohn's disease, cells obtained from histologically inflamed mucosa released significantly more 51Cr [9.7 (2.5%) n = 11] than those from non-inflamed mucosa [6.4 (1.5%) n = 7, p less than 0.02] whereas, in ulcerative colitis, abnormal release values were found in 8 of 13 cell populations isolated from mucosa showing no histological evidence of active disease. In five patients with distal ulcerative colitis, cells from mucosa not apparently involved demonstrated normal 51Cr release in four of five studies despite abnormal release from cells from involved mucosa suggesting that a diffuse abnormality of the colonic epithelial cell is not usually present. These data indicate that chronic mucosal inflammation per se is associated with abnormalities of the colonic epithelial cell but that, in ulcerative colitis, the abnormality remains in many patients with quiescent disease. Identification of the local factors responsible for such an abnormality may contribute to an understanding of the pathogenesis of ulcerative colitis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Baral B., Das K. M. Isolation and characterization of Crohn's disease tissue-specific glycoproteins. Gastroenterology. 1986 Aug;91(2):326–332. doi: 10.1016/0016-5085(86)90564-0. [DOI] [PubMed] [Google Scholar]
- Bleiberg H., Mainguet P., Galand P., Chretien J., Dupont-Mairesse N. Cell renewal in the human rectum. In vitro autoradiographic study on active ulcerative colitis. Gastroenterology. 1970 Jun;58(6):851–855. [PubMed] [Google Scholar]
- Carlsson H. E., Hammarström S., Lanteli M., Perlmann P. Antibody-induced destruction of colon antigen-coated chicken erythrocytes by normal human lymphocytes. Eur J Immunol. 1971 Aug;1(4):281–285. doi: 10.1002/eji.1830010413. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M., Amar J., Gardiner G. Crypt production in normal and diseased human colonic epithelium. Anat Rec. 1986 Sep;216(1):44–48. doi: 10.1002/ar.1092160108. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Epithelial cell renewal in cultured rectal biopsies in ulcerative colitis. Gastroenterology. 1973 Mar;64(3):383–390. [PubMed] [Google Scholar]
- Elson C. O., Kagnoff M. F., Fiocchi C., Befus A. D., Targan S. Intestinal immunity and inflammation: recent progress. Gastroenterology. 1986 Sep;91(3):746–768. doi: 10.1016/0016-5085(86)90649-9. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Reilly R. W., Rosenberg I. H. Small intestinal injury in the graft versus host reaction: an innocent bystander phenomenon. Gastroenterology. 1977 May;72(5 Pt 1):886–889. [PubMed] [Google Scholar]
- Gibson P. R., Jewell D. P. Local immune mechanisms in inflammatory bowel disease and colorectal carcinoma. Natural killer cells and their activity. Gastroenterology. 1986 Jan;90(1):12–19. doi: 10.1016/0016-5085(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Hogan P. G., Hapel A. J., Doe W. F. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J Immunol. 1985 Sep;135(3):1731–1738. [PubMed] [Google Scholar]
- James S. P., Strober W. Cytotoxic lymphocytes and intestinal disease. Gastroenterology. 1986 Jan;90(1):235–237. doi: 10.1016/0016-5085(86)90098-3. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Rampton D. S., Sladen G. E., Youlten L. J. Rectal mucosal prostaglandin E2 release and its relation to disease activity, electrical potential difference, and treatment in ulcerative colitis. Gut. 1980 Jul;21(7):591–596. doi: 10.1136/gut.21.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E., Lawson M. J., Nance S. H., Radcliffe B. C. Detectable colonic nitrite levels in inflammatory bowel disease--mucosal or bacterial malfunction? Digestion. 1986;35(4):199–204. doi: 10.1159/000199368. [DOI] [PubMed] [Google Scholar]
- Roediger W. E., Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol. 1986 Dec;67(6):773–782. [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E., Radcliffe B. C., Deakin E. J., Nance S. H. Specific metabolic effect of sodium nitrite on fat metabolism by mucosal cells of the colon. Dig Dis Sci. 1986 May;31(5):535–539. doi: 10.1007/BF01320321. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter R. G., Huizenga K. A., Spencer R. J., Guy S. K. Inflammatory bowel disease. The role of lymphotoxin in the cytotoxicity of lymphocytes for colonic epithelial cells. Am J Dig Dis. 1972 Aug;17(8):689–696. doi: 10.1007/BF02231636. [DOI] [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Takahashi F., Das K. M. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J Clin Invest. 1985 Jul;76(1):311–318. doi: 10.1172/JCI111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]


