Abstract
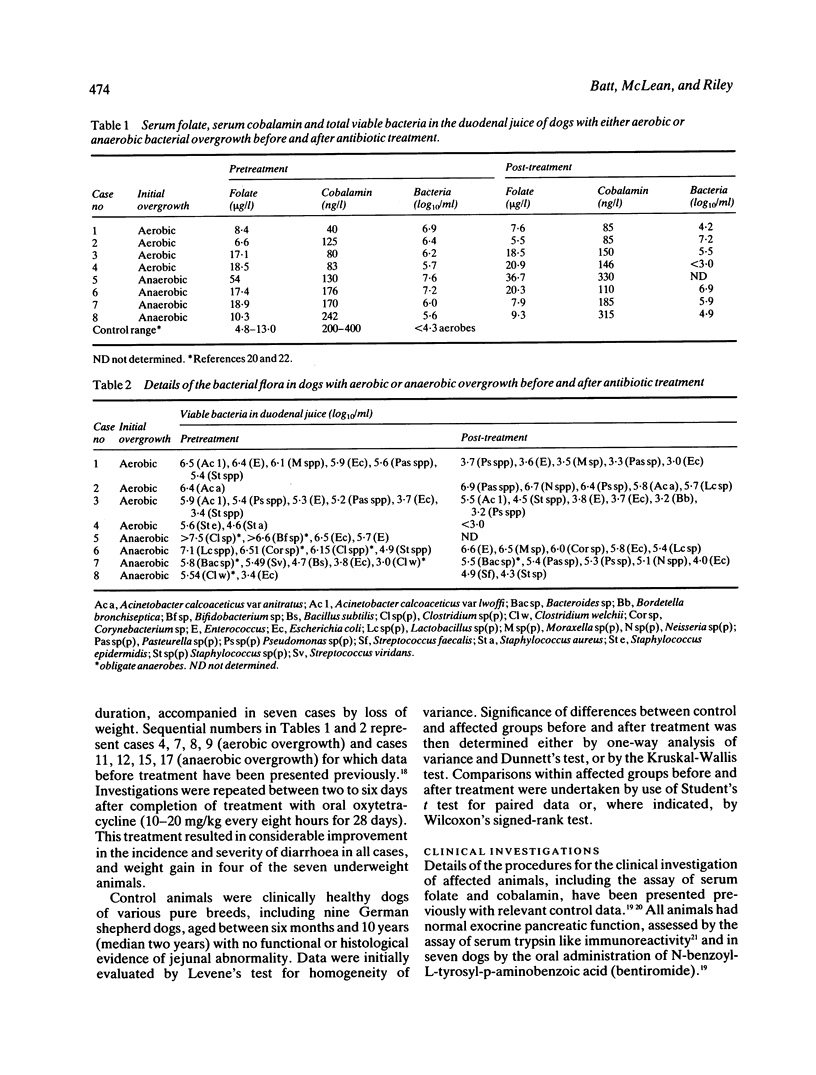
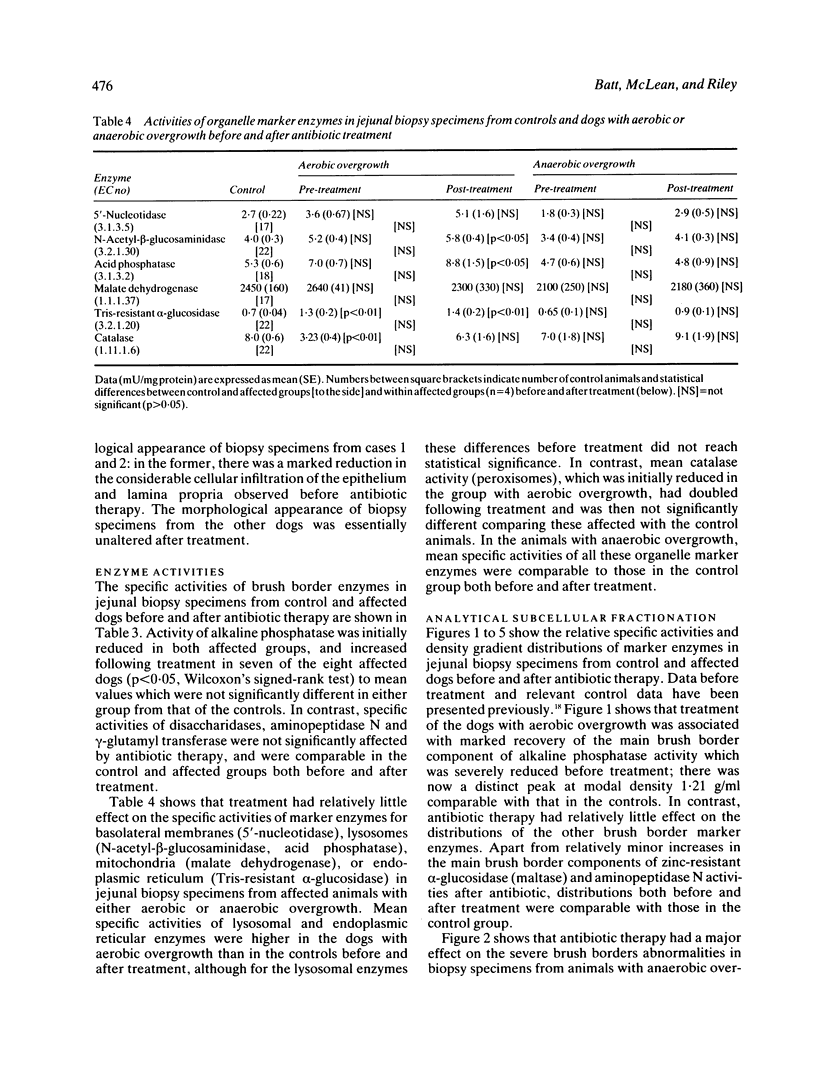
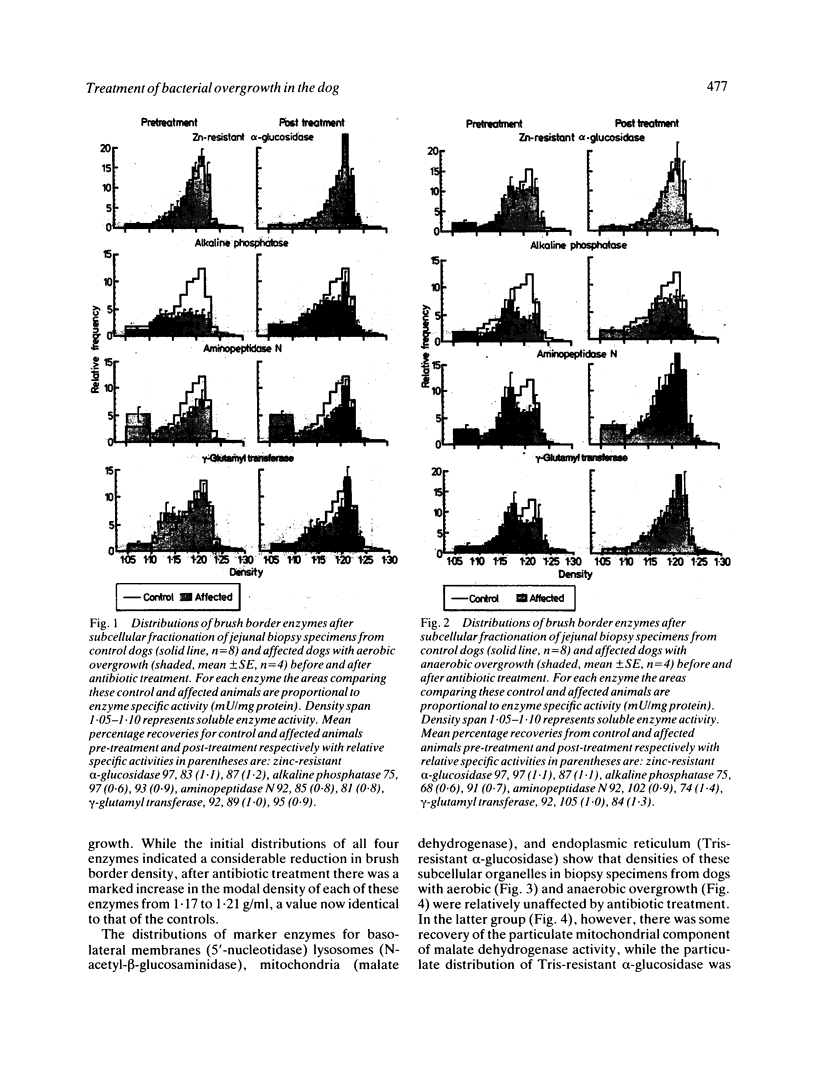
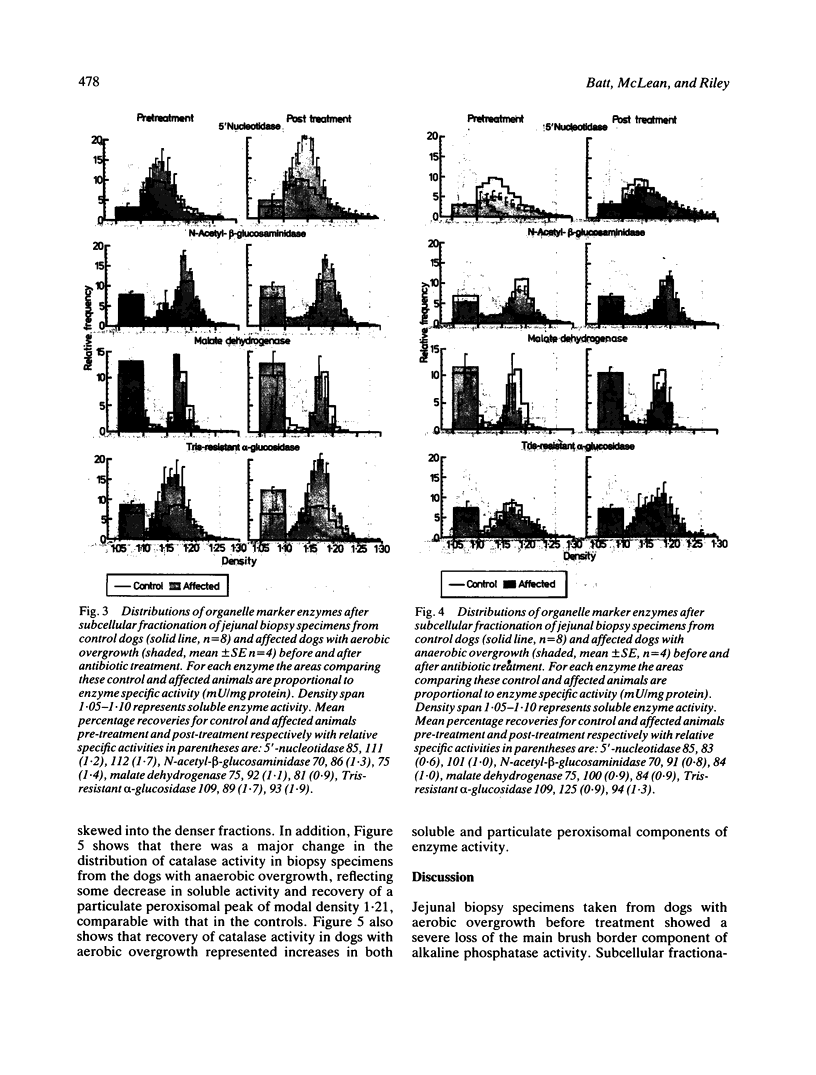
Dogs with naturally occurring aerobic or anaerobic bacterial overgrowth have been examined before and after antibiotic therapy in order to assess reversibility of damage to the jejunal mucosa. Histological changes in peroral jejunal biopsies were relatively minor before and after treatment, but sucrose density gradient centrifugation revealed specific biochemical abnormalities that responded to antibiotic therapy. Aerobic overgrowth was initially associated with a marked loss of the main brush border component of alkaline phosphatase activity; this recovered following treatment, suggesting that aerobic bacteria may cause reversible damage to the hydrophobic region of the brush border membrane. In contrast, anaerobic overgrowth was initially associated with a marked reduction in brush border density, indicative of a considerable fall in the glycoprotein-to-lipid ratio of the membrane. Density increased from 1.17 to 1.21 g/ml after antibiotic therapy, consistent with recovery from this relatively severe damage to the brush border caused by anaerobic bacteria. Reductions in soluble and peroxisomal catalase activities which could compromise mucosal protection against free radicals in dogs with aerobic overgrowth, and a loss of particulate malate dehydrogenase activity indicative of mitochondrial disruption in dogs with anaerobic overgrowth, were also reversed after treatment. These findings indicate that aerobic and anaerobic bacterial overgrowth can result in contrasting but potentially reversible damage to the jejunal mucosa which would not be detected by conventional investigative procedures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ament M. E., Shimoda S. S., Saunders D. R., Rubin C. E. Pathogenesis of steatorrhea in three cases of small intestinal stasis syndrome. Gastroenterology. 1972 Nov;63(5):728–747. [PubMed] [Google Scholar]
- Baraona E., Julkunen R., Tannenbaum L., Lieber C. S. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986 Jan;90(1):103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Bush B. M., Peters T. J. Biochemical changes in the jejunal mucosa of dogs with naturally occurring exocrine pancreatic insufficiency. Gut. 1979 Aug;20(8):709–715. doi: 10.1136/gut.20.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt R. M., Carter M. W., Peters T. J. Biochemical changes in the jejunal mucosa of dogs with a naturally occurring enteropathy associated with bacterial overgrowth. Gut. 1984 Aug;25(8):816–823. doi: 10.1136/gut.25.8.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt R. M., Mann L. C. Specificity of the BT-PABA test for the diagnosis of exocrine pancreatic insufficiency in the dog. Vet Rec. 1981 Apr 4;108(14):303–307. doi: 10.1136/vr.108.14.303. [DOI] [PubMed] [Google Scholar]
- Batt R. M., McLean L., Carter M. W. Sequential morphologic and biochemical studies of naturally occurring wheat-sensitive enteropathy in Irish setter dogs. Dig Dis Sci. 1987 Feb;32(2):184–194. doi: 10.1007/BF01297107. [DOI] [PubMed] [Google Scholar]
- Batt R. M., McLean L. Comparison of the biochemical changes in the jejunal mucosa of dogs with aerobic and anaerobic bacterial overgrowth. Gastroenterology. 1987 Nov;93(5):986–993. doi: 10.1016/0016-5085(87)90560-9. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Morgan J. O. Role of serum folate and vitamin B12 concentrations in the differentiation of small intestinal abnormalities in the dog. Res Vet Sci. 1982 Jan;32(1):17–22. [PubMed] [Google Scholar]
- Batt R. M., Needham J. R., Carter M. W. Bacterial overgrowth associated with a naturally occurring enteropathy in the German shepherd dog. Res Vet Sci. 1983 Jul;35(1):42–46. [PubMed] [Google Scholar]
- Batt R. M., Peters T. J. Subcellular fractionation studies on peroral jejunal biopsies from the dog. Res Vet Sci. 1978 Jul;25(1):94–100. [PubMed] [Google Scholar]
- Batt R. M. Techniques for single and multiple peroral jejunal biopsy in the dog. J Small Anim Pract. 1979 May;20(5):259–268. doi: 10.1111/j.1748-5827.1979.tb06720.x. [DOI] [PubMed] [Google Scholar]
- Bloch R., Menge H., Lorenz-Meyer H., Stöckert H. G., Riecken E. O. Functional, biochemical and morphological alterations in the intestines of rats with an experimental blind-loop syndrome. Res Exp Med (Berl) 1975 Nov 26;166(1):67–78. doi: 10.1007/BF01851347. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Rubin E., Cohen G. The effect of dimethylsulfoxide and other hydroxyl radical scavengers on the oxidation of ethanol by rat liver microsomes. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1254–1262. doi: 10.1016/0006-291x(77)91428-0. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Maroux S. Integration of alkaline phosphatase in the intestinal brush border membrane. Biochim Biophys Acta. 1978 Jul 20;511(1):39–51. doi: 10.1016/0005-2736(78)90063-9. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Toskes P. P. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974 Nov;67(5):965–974. [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Jacobs B., Chatterjee B. D., Mitra R., Mazumder D. N., Sen N. N. Tropical sprue and malnutrition in West Bengal. I. Intestinal microflora and absorption. Am J Clin Nutr. 1970 Dec;23(12):1545–1558. doi: 10.1093/ajcn/23.12.1545. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L. Intestinal microflora. Gastroenterology. 1971 Jun;60(6):1110–1129. [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A., Barker J., Glasgow E. F. Bacteria, bile salts, and intestinal monosaccharide malabsorption. Gut. 1971 Sep;12(9):683–692. doi: 10.1136/gut.12.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M., Papadimitriou J., Bower G. Ultrastructural changes in the small intestines of rats with self-filling blind loops. Gastroenterology. 1974 Oct;67(4):646–651. [PubMed] [Google Scholar]
- Gracey M., Thomas J., Houghton M. Effect of stasis on intestinal enzyme activities. Aust N Z J Med. 1975 Apr;5(2):141–144. doi: 10.1111/j.1445-5994.1975.tb03643.x. [DOI] [PubMed] [Google Scholar]
- Hansson R., Johansson S., Jonsson O., Pettersson S., Scherstén T., Waldenström J. Kidney protection by pretreatment with free radical scavengers and allopurinol: renal function at recirculation after warm ischaemia in rabbits. Clin Sci (Lond) 1986 Sep;71(3):245–251. doi: 10.1042/cs0710245. [DOI] [PubMed] [Google Scholar]
- Jonas A., Flanagan P. R., Forstner G. G. Pathogenesis of mucosal injury in the blind loop syndrome. Brush border enzyme activity and glycoprotein degradation. J Clin Invest. 1977 Dec;60(6):1321–1330. doi: 10.1172/JCI108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A., Krishnan C., Forstner G. Pathogenesis of mucosal injury in the blind loop syndrome. Gastroenterology. 1978 Nov;75(5):791–795. [PubMed] [Google Scholar]
- King C. E., Toskes P. P. Small intestine bacterial overgrowth. Gastroenterology. 1979 May;76(5 Pt 1):1035–1055. [PubMed] [Google Scholar]
- Klipstein F. A., Holdeman L. V., Corcino J. J., Moore W. E. Enterotoxigenic intestinal bacteria in tropical sprue. Ann Intern Med. 1973 Nov;79(5):632–641. doi: 10.7326/0003-4819-79-5-632. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayudu P. R., Hercus F. B. Molecular heterogeneity of mouse duodenal alkaline phosphatase. Association of lipids and peptides. Biochem J. 1974 Jul;141(1):93–101. doi: 10.1042/bj1410093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Batt R. M., Heath J. R., Tilleray J. The micro-assay of intestinal disaccharidases. Biochem Med. 1976 Apr;15(2):145–148. doi: 10.1016/0006-2944(76)90041-7. [DOI] [PubMed] [Google Scholar]
- Prizont R. Glycoprotein degradation in the blind loop syndrome: identification of glycosidases in jejunal contents. J Clin Invest. 1981 Feb;67(2):336–344. doi: 10.1172/JCI110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepe S. P., Goldstein J., Alpers D. H. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest. 1980 Aug;66(2):314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Sherman P., Wesley A., Forstner G. Sequential disaccharidase loss in rat intestinal blind loops: impact of malnutrition. Am J Physiol. 1985 Jun;248(6 Pt 1):G626–G632. doi: 10.1152/ajpgi.1985.248.6.G626. [DOI] [PubMed] [Google Scholar]
- Small intestinal bacterial overgrowth syndrome. Gastroenterology. 1981 Apr;80(4):834–845. [PubMed] [Google Scholar]
- Solimano G., Burgess E. A., Levin B. Protein-calorie malnutrition: effect of deficient diets on enzyme levels of jejunal mucosa of rats. Br J Nutr. 1967;21(1):55–68. doi: 10.1079/bjn19670009. [DOI] [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Troglia O. M., Laughrey E. G., Henley K. S. Effect of quantitative undernutrition on the activities of intestinal disaccharidases in the rat. Gastroenterology. 1970 May;58(5):669–672. [PubMed] [Google Scholar]


