Abstract
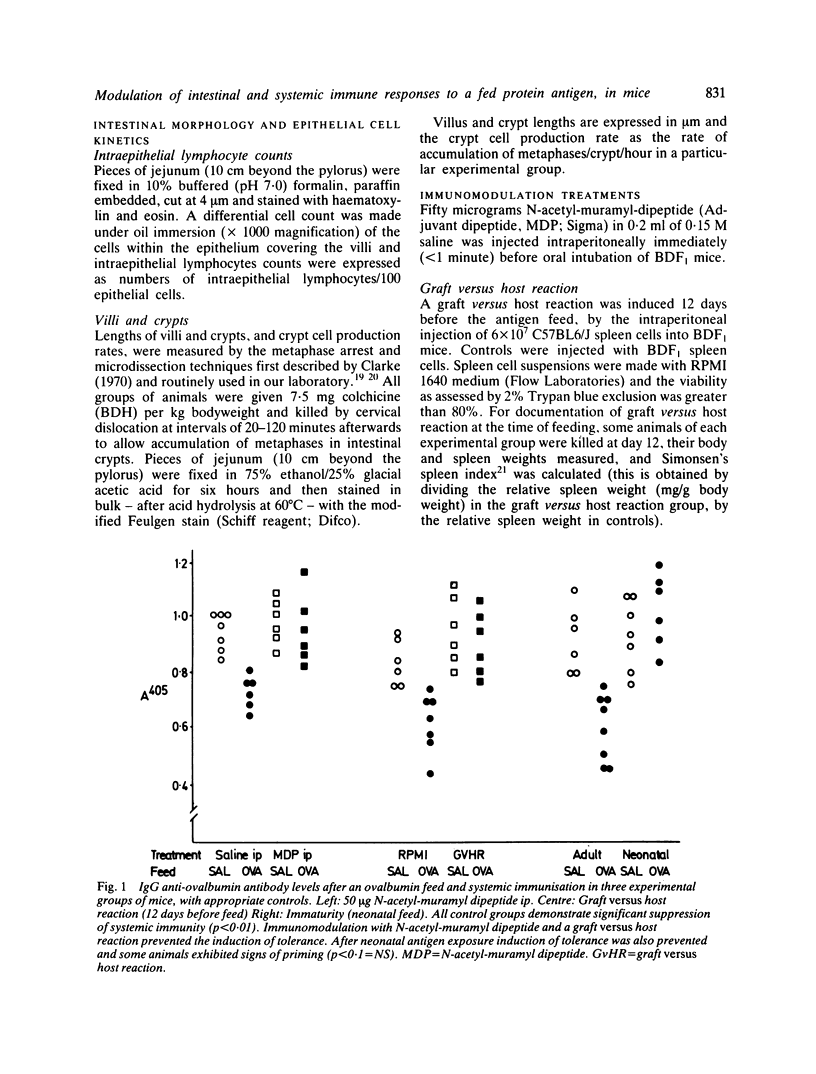
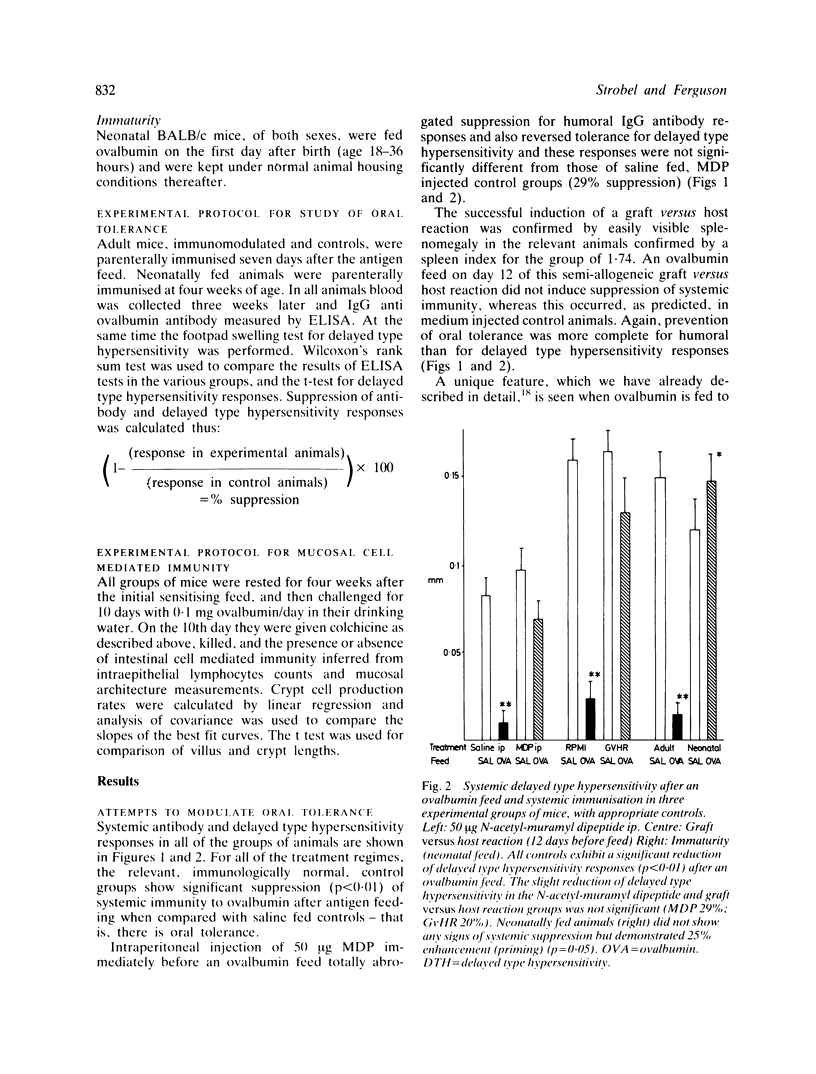
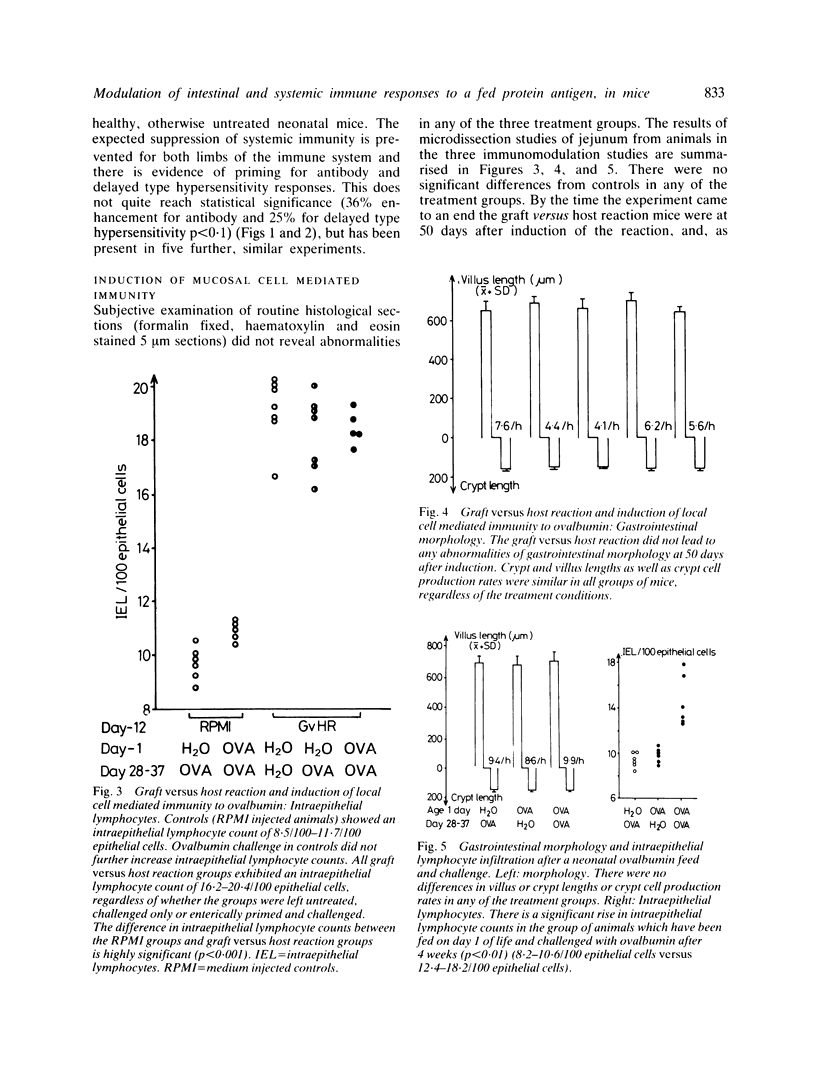
Feeding of a protein antigen to normal adult mice results in systemic immunologic hyporesponsiveness (oral tolerance). Local mucosal cell mediated immunity is not usually elicited. The objectives of these experiments were to abrogate the induction of oral tolerance and concomitantly to induce a local mucosal cell mediated immune response in mice; and thereby to establish which facets of intestinal physiology or immunology are relevant to the induction of normal, mainly suppressor immune responses to a fed antigen. Protein antigen (ovalbumin) was fed to animals in whom immune status had been modulated by intraperitoneal injection of N-acetyl-muramyl dipeptide (MDP), by induction of a graft versus host reaction, or naturally, by virtue of immaturity. The induction of oral tolerance was prevented in all treatment groups. In a second series of experiments mice were orally immunised as before, rested for four weeks, and then challenged with ovalbumin in their drinking water for 10 days. Jejunal architecture was not altered by the antigen challenge, but MDP treated and immature animals which had been sensitised to ovalbumin and later re-exposed to the same antigen had significantly higher intraepithelial lymphocyte counts than appropriate controls. Factors which may lead to abrogation of oral tolerance and induction of intestinal hypersensitivity are discussed in relation to food allergic diseases in man.
Full text
PDF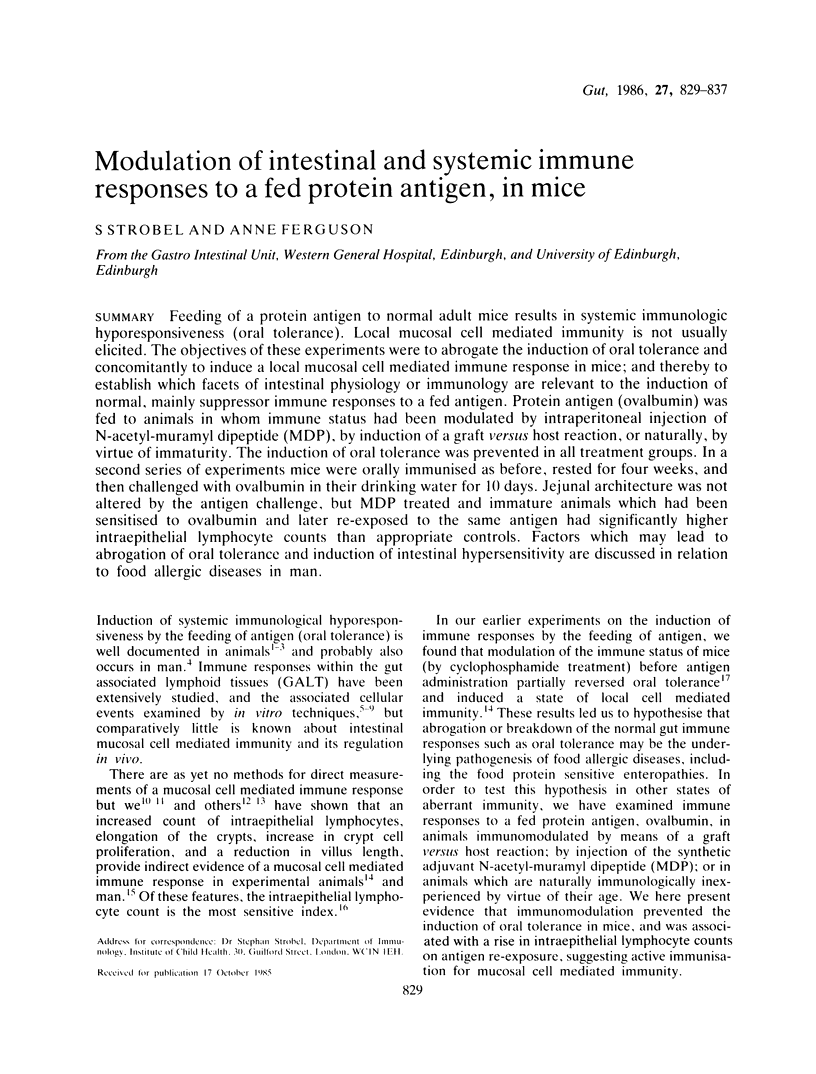




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. G. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J Immunol. 1981 Oct;127(4):1518–1524. [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Hornbrook M. M., Lynch J. M., Roy C. A. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55(1-6):526–532. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- Jenkins H. R., Pincott J. R., Soothill J. F., Milla P. J., Harries J. T. Food allergy: the major cause of infantile colitis. Arch Dis Child. 1984 Apr;59(4):326–329. doi: 10.1136/adc.59.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Kagnoff M. F. Nonspecific recruitment of cytotoxic effector cells in the intestinal mucosa of antigen-primed mice. J Exp Med. 1984 Dec 1;160(6):1931–1936. doi: 10.1084/jem.160.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenblat P. E., Rothberg R. M., Minden P., Farr R. S. Immune responses of human adults after oral and parenteral exposure to bovine serum albumin. J Allergy. 1968 Apr;41(4):226–235. doi: 10.1016/0021-8707(68)90046-4. [DOI] [PubMed] [Google Scholar]
- Kuitunen P., Visakorpi J. K., Savilahti E., Pelkonen P. Malabsorption syndrome with cow's milk intolerance. Clinical findings and course in 54 cases. Arch Dis Child. 1975 May;50(5):351–356. doi: 10.1136/adc.50.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwy I., Bona C., Chedid L. Target cells for the activity of a synthetic adjuvant: muramyl dipeptide. Cell Immunol. 1977 Mar 1;29(1):195–199. doi: 10.1016/0008-8749(77)90288-x. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. II. Antigen-specific helper and suppressor factors produced by spleen cells of rats fed sheep erythrocytes. J Immunol. 1980 Sep;125(3):1044–1047. [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. II. Characterization of the suppression cell and its mechanism of action. Immunology. 1976 Dec;31(6):943–951. [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Oral tolerance--induction and modulation. Klin Padiatr. 1985 Jul-Aug;197(4):297–301. doi: 10.1055/s-2008-1033987. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Chiller J. M. Orally induced tolerance. Definition at the cellular level. Int Arch Allergy Appl Immunol. 1981;65(3):323–338. [PubMed] [Google Scholar]
- Treiber W., Lapp W. S. Experimental stimulation of cell-mediated immunity without concomitant stimulation of humoral immunity in graft-versus-host immunosuppressed mice. Transplantation. 1976 May;21(5):391–398. doi: 10.1097/00007890-197605000-00006. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the induction of suppressor T cells of delayed footpad reaction to SRBC in mice. Immunology. 1981 Jun;43(2):241–247. [PMC free article] [PubMed] [Google Scholar]


