Abstract
Cell-mediated immune (CMI) responses and tumor necrosis factor alpha (TNF-α) have been shown to be essential in acquired protection against Cryptococcus neoformans. Induction of a protective anticryptococcal CMI response includes increases in dendritic cells (DC) and activated CD4+ T cells in draining lymph nodes (DLN). During the expression phase, activated CD4+ T cells accumulate at a peripheral site where cryptococcal antigen is injected, resulting in a classical delayed-type hypersensitivity (DTH) reaction. Induction of a nonprotective anticryptococcal CMI response results in no significant increases in the numbers of DC or activated CD4+ T cells in DLN. This study focuses on examining the role of TNF-α in induction of protective and nonprotective anticryptococcal CMI responses. We found that neutralization of TNF-α at the time of immunization with the protective immunogen (i) reduces the numbers of Langerhans cells, myeloid and lymphoid DC, and activated CD4+ T cells in DLN and (ii) diminishes the total numbers of cells, the numbers of activated CD4+ T cells, and amount of gamma interferon at the DTH reaction site. Although TNF-α neutralization during induction of the nonprotective CMI response had little effect on cellular and cytokine parameters measured, it did cause a reduction in footpad swelling when mice received challenge in the footpad. Our findings show that TNF-α functions during induction of the protective CMI response by influencing the accumulation of all three DC subsets into DLN. Without antigen stimulated DC in DLN, activated CD4+ T cells are not induced and thus not available for the expression phase of the CMI response.
Frequently, fatal meningitis in immunocompetent and immunocompromised adults is caused by Cryptococcus neoformans, a yeast-like organism (18). Protection against C. neoformans is mediated by an anticryptococcal cell-mediated immune (CMI) response (19). For this acquired immune response to be protective, activated CD4+ Th1 cells and gamma interferon (IFN-γ) must be present (1, 6, 12, 14, 17, 21, 29). In addition, the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) has been shown to be required during the early stages of infection with C. neoformans for mice to clear the infection (15). Without a CMI response against C. neoformans, as is the case in immunocompromised patients, the host is unable to completely clear C. neoformans even when the best antifungal drugs are being administered (5). Consequently, immunoaugmenting and immunoreplacement therapy needs to be developed; however, to do this, the complex cascade of cell-cell and cytokine-cell interactions involved in the anticryptococcal CMI response must be completely understood.
As a means of studying this complex cascade of cells and cytokines, we have developed mouse immunization procedures that induce either a protective anticryptococcal CMI response or a nonprotective immune response. Both the protective and nonprotective immune responses can be detected by delayed footpad swelling in response to cryptococcal antigen (23). Neither immunogen induces measurable levels of anticryptococcal antibody (23). Using this model, we have recently demonstrated that the protective acquired response induced by immunization with cryptococcal culture filtrate (CneF) antigen in complete Freund's adjuvant (CneF-CFA) is a typical CMI response giving a classical delayed-type hypersensitivity (DTH) reaction consisting of mononuclear cell infiltrates and elevated IFN-γ levels at the DTH reaction site (25). In contrast, the delayed-swelling reaction in mice immunized with the nonprotective immunogen, heat-killed C. neoformans (HKC) in CFA (HKC-CFA), has increased neutrophil, but not lymphocyte or macrophage, numbers above control levels (25). Our previous results demonstrate that during the expression phase of the protective CMI response, cellular and cytokine responses are clearly different from those observed during the expression phase of the nonprotective response (25).
We have also studied the induction phase of the anticryptococcal CMI response and found that within 18 h after immunizing mice with the protective immunogen, the numbers of dendritic cells (DC) and activated CD4+ T cells are increased significantly in the draining lymph nodes (DLN), but not after immunizing with the nonprotective immunogen (1). Considering (i) that TNF-α is necessary early in a cryptococcal infection for protection to be observed (15) and (ii) that TNF-α has been shown to be needed for the migration of DC into lymph nodes after stimulation with contact sensitins and for expression of a DTH response to the sensitins (7, 26), we hypothesized that TNF-α influences the induction phase of the protective but not the nonprotective anticryptococcal acquired immune response by affecting the numbers and possibly the phenotype(s) of DC infiltrating into lymph nodes draining the immunization site. If this hypothesis is true, then neutralization of TNF-α with anti-TNF-α antibody in vivo at the time of immunization with the protective cryptococcal immunogen, CneF-CFA, would affect the whole anticryptococcal CMI cascade, beginning with a reduction in the number of DC in the DLN. A domino effect would follow, resulting in diminished numbers of activated CD4+ T cells in the DLN, fewer activated CD4+ T cells available to cause a strong DTH reaction, diminished numbers of activated CD4+ T cells at the DTH reaction site, and low levels of IFN-γ at the DTH reaction site. In contrast, neutralization of TNF-α during the induction phase of the nonprotective anticryptococcal response would be expected to have little effect, because significant numbers of DC and activated CD4+ T cells do not accumulate in the DLN. The purpose of this study was to test these predictions.
MATERIALS AND METHODS
Mice.
Female CBA/J mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and maintained in the animal facility at the University of Oklahoma Health Sciences Center. Three to five mice per group were used in all experiments. The mice were 7 to 10 weeks of age at the beginning of each experiment.
Maintenance of endotoxin-free conditions.
All reagents for injection into animals were tested for endotoxin content and were not used if the endotoxin level was detectable by the Limulus amebocyte chromogenic assay (BioWhittaker) (the minimal detectable level of endotoxin was 0.1 ng of endotoxin/ml). Sterile tissue culture plasticware was used whenever possible. All glassware was baked at 180°C for 3 h to destroy endotoxin.
Cryptococcal antigens and immunization.
CneF from C. neoformans isolate 184A was prepared as previously described (2). HKC were prepared by incubating C. neoformans isolate 184A for 1 h at 60°C (24). Mice received subcutaneous injections at two sites at the base of the tail with 0.2 ml of either a 1:1 emulsion of CneF in CFA or 107 HKC in CFA or sterile physiological saline in CFA (24). In experiments in which DTH reaction measurements were not the primary reason for the experiments, five additional mice were immunized with each of the immunogens to confirm that appropriate induction of DTH responses had been accomplished.
Detection of anticryptococcal DTH responsiveness.
At 7 days after immunization, the hind footpads of the mice were measured, and then mice received injections with 30 μl of saline in the left footpad and 30 μl of CneF in the right footpad. The footpads were measured 24 h after the challenge injection. The increase in footpad thickness was calculated by subtracting the difference in swelling in the 0- and 24-h measurements of the saline-injected footpad from the difference in swelling between the 0- and 24-h measurements of the CneF-injected footpads.
TNF-α neutralization.
To neutralize TNF-α during the induction phase studies, 8 h before immunization mice received intraperitoneal (i.p.) injections with 250 μg of anti-TNF-α monoclonal antibody (MAb) (MP6-XT3; kindly provided by R. Coffman, DNAX) (15) or rat immunoglobulin G (IgG) (Cappell). To neutralize TNF-α during the expression phase, mice received injections with 250 μg of anti-TNF-α MAb or rat IgG 8 h before footpad challenge on day 7 after immunization.
Preparation of single-cell suspensions.
Lymph nodes (superficial inguinal, lumbar, and popliteal) were removed at 18 h after immunization and pushed through a wire mesh screen to prepare single-cell suspensions. The screens and cell suspensions were incubated for 30 min on ice in collagenase D (100 U/ml; Boehringer Mannheim) in Hanks balanced salt solution (HBSS)-5% fetal calf serum. The cell suspensions were washed and resuspended in HBSS-5% fetal calf serum. This method of cell isolation was selected because it is considered best for extracting all populations of DC (1, 27, 28), and DC populations were the cells of primary interest in this study. Furthermore, in the preparation of the cell suspensions we avoided culture steps that might change the DC to a mature phenotype.
Sponge implantation and injection with antigen.
To have DTH reaction sites for which the infiltrating cell populations and cytokines can be studied, gelatin sponges (Gelfoam sterile absorbable gelatin sponge; Upjohn Co., Kalamazoo, Mich.) were surgically implanted under aseptic conditions on either side of the backs of the mice (2). The sponge blocks (17 by 18 by 10 mm) hydrated with sterile HBSS containing 100 U of penicillin/ml and 100 mg of streptomycin/ml were implanted subcutaneously. through an incision on the animals' shaved backs on day 3 after immunization with CneF-CFA or HKC-CFA or control treatment. Four days after implantation, one sponge was injected with 0.1 ml of CneF and the other was injected with 0.1 ml of saline. The sponges were removed at 24 h after injection.
Sponge retrieval and disaggregation.
Mice were euthanized before surgical removal of sponges. Fluid was expressed from the sponges and stored at −70°C until used to determine IFN-γ levels. Sponges were put into Stomacher bags (Tekmar Co., Cincinnati, Ohio) with enzyme cocktail (400 U of collagenase/ml; Sigma Co., St. Louis, Mo.) and then homogenized with three 10-s pulses on a Stomacher 80 Lab Blender (Tekmar Co.) at 15-min intervals (8). During the 15-min intervals, the sponge homogenates were incubated at 37°C. Following the disaggregation step, the sponge homogenates were filtered through 390-μm-pore-size nylon screens followed by passage through 140 μm nylon screens, and washed with HBSS. The erythrocytes in the sponge homogenates were lysed by treatment with Tris-NH4Cl (17 mM Tris, 139.7 mM NH4Cl), and the remaining cells were washed once with HBSS. Viable cell counts were made using trypan blue dye exclusion and a hemacytometer.
Flow cytometric analysis.
Portions of the lymph node single-cell suspensions from individual mice (three mice per group) were immunolabeled with the designated MAbs. For the isotype control, another portion of each cell suspension was pooled within a treatment group before being carried through the staining procedure. Individual cell suspensions and pooled cell suspensions were treated with anti-CD16/32 (HB197; American Type Culture Collection [ATCC]) for 30 min at 4°C to block Fc receptors and thereby reduce nonspecific staining of cells. After centrifugation, cells were incubated for 30 min at 4°C in wash buffer (phosphate-buffered saline, 0.1% NaN3, 0.1% bovine serum albumin) containing fluorochrome-labeled MAbs or isotype control MAbs. After washing, the cells were fixed with 1% paraformaldehyde. The antibodies used in these studies included DEC205-fluorescein isothiocyanate (NLDC-145; ATCC), CD8α-allophycocyanin (CT-CD8a; Caltag), CD4-fluorescein isothiocyanate (CT-CD4; Caltag), CD45RB-phycoerythrin (16A; Pharmingen), and fluorochrome-labeled isotype control MAbs(Caltag). After fixing, 100,000 to 150,000 cells were analyzed using a FACSCalibur flow cytometer and WinMidi 2.8 software. Identification of the three DC subsets—Langerhans cells, myeloid DC (MDC), and lymphoid DC (LDC)-was done on the basis of the criteria previously described (1). Briefly, the forward scatter high (FSChi) cells were divided into FSChi DEC205hi and FSChi DEC205low. The Langerhans cells were the FSChi DEC205low cells (1). The FSChi DEC205hi cells were further divided into FSChi DEC205hi CD8αlow, representing the MDC, and FSChiDEC205hiCD8αhi, representing the LDC (1). The percent positive cells were the percentage of the designated cell population with fluorescent intensity above the fluorescent intensity of cells treated with isotype control MAbs. The total number of positive cells in each sample was determined by multiplying the percent positive cells for the sample by the total number of leukocytes in the sample, which was assessed by hemocytometer counts.
Quantitation of IFN-γ levels in sponge supernatants.
An enzyme-linked immunosorbent assay (ELISA) for the detection of IFN-γ in expressed sponge fluid was constructed using commercially available paired monoclonal antibodies for IFN-γ (PharMingen) according to our previously described method (20). The minimal level of detection for the IFN-γ ELISA was 62.5 pg/ml.
Statistical analysis.
Means and standard error of the means (SEM) were determined for each group. Analysis of variance with a Newman-Keuls posttest was used to analyze the data. A P of ≤0.05 was considered to be significant.
RESULTS
Effect of TNF-α neutralization on lymph node cellularity, DC populations, and activated CD4+ T cells in mice immunized with the protective or nonprotective cryptococcal immunogen or saline-CFA.
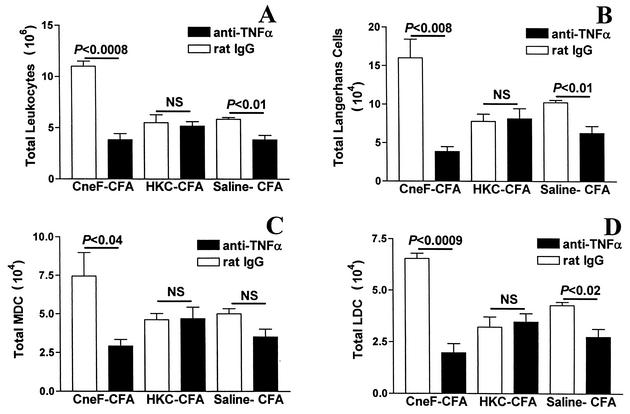
We neutralized TNF-α with anti-TNF-α MAb at the time of immunization with the protective cryptococcal immunogen, CneF-CFA, and found that the total numbers of leukocytes in the DLN 18 h after immunization were significantly reduced compared to numbers in DLN of mice given rat IgG in place of the TNF-α-neutralizing MAb (Fig. 1A). Furthermore, CneF-CFA-immunized mice that had TNF-α neutralized had significantly fewer Langerhans cells (Fig. 1B), MDC (Fig. 1C), and LDC (Fig. 1D) in DLN than were seen in DLN of mice immunized with CneF-CFA and given rat IgG.
FIG. 1.
Effect of TNF-α neutralization on numbers of leukocytes, Langerhans cells, MDC, and LDC in DLN in response to cryptococcal immunization. Mice received i.p. injections with 250 μg of either anti-TNF-α MAb or rat IgG 8 h before immunization. DLN were removed from mice at 18 h after immunization with CneF-CFA or HKC-CFA or treatment with saline-CFA. After collagenase digestion of the lymph nodes, the numbers of leukocytes were assessed by hemocytometer counts (A), and cells were stained for Langerhans cells (DEC205low FSChi) (B), MDC (DEC205hi FSChi CD8αlow) (C), and LDC (DEC205hi FSChi CD8αhi) (D) as described previously (1). The stained cells were analyzed by three-color flow cytometric analysis of 100,000 to 150,000 events. Error bars represent SEM. NS, no significant difference.
TNF-α neutralization had no effect on the numbers of leukocytes or DC subsets in DLN of mice immunized with the nonprotective cryptococcal immunogen, HKC-CFA. This was evident because the total number of leukocytes and DC subsets in the mice immunized with HKC-CFA and treated with neutralizing anti-TNF-α MAb were similar to numbers of the respective cell population in DLN of mice immunized with HKC-CFA and treated with rat IgG (Fig. 1).
Our data show that TNF-α neutralization in mice immunized with saline-CFA results in significantly reduced numbers of total leukocytes (Fig. 1A), Langerhans cells (Fig. 1B), and LDC (Fig. 1D) in DLN compared to equivalent cell populations in DLN of mice immunized with saline-CFA and given rat IgG as a control for the anti-TNF-α MAb. The numbers of leukocytes in DLN of mice immunized with CneF-CFA or saline-CFA and given anti-TNF-α MAb (Fig. 1A) were similar to the number of leukocytes in DLN of naïve mice (3.2 × 106 ± 0.2 × 106). Furthermore, the number of leukocytes in DLN from rat IgG-injected, immunized mice (Fig. 1A) were similar to our previously reported results on the total numbers of leukocytes in DLN of immunized mice not treated with rat IgG (1).
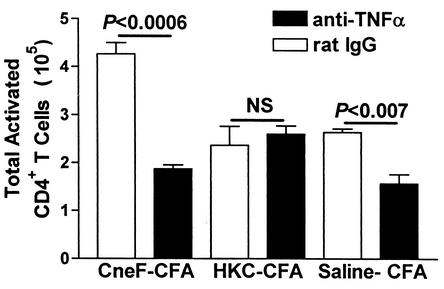
Our previous experience with these immunizations has shown that if an immunization protocol induces increases in the DC populations in DLN then the numbers of activated CD4+ T cells are increased above control levels (1). Consequently, the reduction in DC influx into DLN after immunization with the protective cryptococcal antigen and neutralization of TNF-α would be expected to result in reduced T cell activation. In fact, we observed that TNF-α neutralization in CneF-CFA-immunized mice significantly reduced the number of CD45RBlow (activated phenotype) CD4+ T cells in DLN compared to the number of CD45RBlow CD4+ T cells in rat-IgG-treated, CneF-CFA-immunized mice (Fig. 2). In contrast, TNF-α neutralization did not affect the number of activated CD4+ T cells in DLN from HKC-CFA-immunized mice (Fig. 2) in which there had been no reduction in DC due to TNF-α neutralization (Fig. 1B to D). The number of activated CD4+ T cells was significantly reduced in anti-TNF-α MAb-injected, saline-CFA-treated mice compared to rat-IgG-injected, saline-CFA-treated mice (Fig. 2). The number of activated CD4+ T cells in DLN of rat-IgG-injected, immunized mice (Fig. 2) was similar to the numbers of activated CD4+ T cells found in immunized mice not treated with rat IgG (1).
FIG. 2.
Effect of TNF-α neutralization on numbers of activated CD4+ T cells in lymph nodes of mice immunized with the protective or nonprotective cryptococcal immunogen. Mice received i.p. injections with 250 μg of either anti-TNF-α MAb or rat IgG 8 h before immunization. DLN were removed from mice at 18 h after immunization with CneF-CFA or HKC-CFA or treatment with saline-CFA. After collagenase digestion of the lymph nodes, cells were stained for activated CD4+ T cells. The stained cells were analyzed by two-color flow cytometric analysis of 100,000 to 150,000 events. Activated CD4+ T cells were defined by light scatter and CD4+ CD45RBlow. Error bars represent SEM. NS, no significant difference.
Effects of neutralizing TNF-α either at the time of induction or time of expression of the anticryptococcal CMI response.
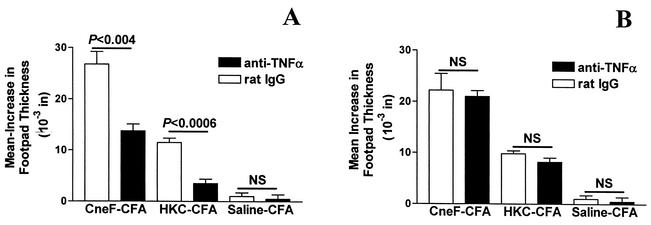
Considering that TNF-α neutralization in CneF-CFA-immunized, but not in HKC-CFA-immunized, mice reduced the number of activated CD4+ T cells produced in DLN, there would be fewer activated CD4+ T cells in circulation in CneF-CFA immunized mice in which TNF-α was neutralized than in mice given control IgG. Reduced numbers of activated CD4+ T cells in DLN after protective immunization have correlated with reduced DTH reactivity (1). When we gave anti-TNF-α MAb at the time of immunization, a significant reduction in footpad swelling at 24 h after CneF injection into the footpads was observed in mice immunized with CneF-CFA and in mice immunized with HKC-CFA (Fig. 3A). Footpads did not show positive delayed swelling in response to CneF in mice immunized with saline-CFA, and treatment of saline-CFA-immunized mice with anti-TNF-α MAb at the time of immunization did not change the negative results, i.e., little or no footpad swelling (Fig. 3A).
FIG. 3.
Effect of TNF-α neutralization on the anticryptococcal DTH response in mice immunized with CneF-CFA or HKC-CFA or treated with saline-CFA. TNF-α neutralization was done by injecting 250 μg of anti-TNF-α MAb i.p., or as a control, mice were given rat IgG i.p. at 8 h before immunization (A) or at 8 h before footpad challenge (B). Error bars represent SEM. NS, no significant difference.
Mice immunized with CneF-CFA produce TNF-α within the DTH reaction site during the expression phase of an anticryptococcal CMI response (3). Therefore, we anticipated that TNF-α neutralization during the expression phase of the anticryptococcal DTH response would also reduce the DTH response. Contrary to our expectations, there was no reduction in the anticryptococcal DTH response in either the CneF-CFA-immunized or HKC-CFA-immunized mice when anti-TNF-α was injected during the expression phase of the immune response (Fig. 3B).
Neutralization of TNF-α at the time of immunization affects cells and cytokines during the expression phase of the anticryptococcal CMI response.
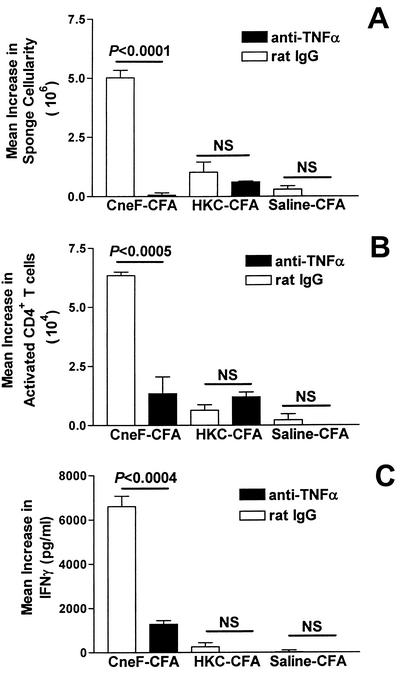
From our earlier studies, we know that activated CD4+ T cells and IFN-γ are significantly increased at the DTH reaction site in mice immunized with the protective immunogen but not in mice immunized with the nonprotective immunogen (25). Having found that neutralization of TNF-α during the induction phase of the anticryptococcal CMI response reduced the production of activated CD4+ T cells in DLN and reduced the level of the anticryptococcal DTH response, we expected to find a reduction in numbers of leukocytes, numbers of activated CD4+ T cells, and IFN-γ levels in the anticryptococcal DTH reaction site. To assess this, the gelatin sponge model was used as had been done previously to define the cells and cytokines at the DTH reaction site (1-4, 9, 10, 25). When injected with recall antigen, the sponges implanted into the backs of immunized mice act as surrogate DTH reaction sites (1-4, 9, 10, 25). Using the sponge model, we found that TNF-α neutralization at the time of immunization of mice with CneF-CFA dramatically reduced the leukocyte influx into the anticryptococcal DTH reaction site, but not into the DTH reaction site in HKC-CFA-immunized mice (Fig. 4A). The reduction in cellularity in the anti-TNF-α-treated mice, in part, could be attributed to the reduced number of activated CD4+ T cells that we observed had migrated into the anticryptococcal DTH reaction site in mice that had been given anti-TNF-α at the time of immunization with CneF-CFA (Fig. 4B). In contrast, TNF-α neutralization had no measurable effect on the minimal influx of CD4+ T cells into DTH reactive sponges in HKC-CFA-immunized or saline-CFA-treated mice (Fig. 4B). Because activated CD4+ T cells are the most likely source of IFN-γ at the DTH reaction site and TNF-α neutralization significantly reduced the number of activated CD4+ T cells at the DTH reaction site in CneF-CFA-immunized mice, but not in HKC-CFA-immunized mice (Fig. 4B), we expected TNF-α neutralization to reduce the amount of IFN-γ at the DTH reaction site in mice immunized with CneF-CFA, but not in mice immunized with HKC-CFA. Indeed, TNF-α neutralization at the time of immunization significantly reduced the amount of IFN-γ at the anticryptococcal DTH reaction site in CneF-CFA-immunized mice, but not at the DTH reaction site in HKC-CFA-immunized or saline-CFA-treated mice (Fig. 4C), confirming our predictions.
FIG. 4.
Effect of TNF-α neutralization on the numbers of leukocytes and activated CD4+ T cells and level of IFN-γ at the anticryptococcal DTH reaction site in mice immunized with CneF-CFA or HKC-CFA or treated with saline-CFA. TNF-α neutralization was done by injecting 250 μg of anti-TNF-α MAb i.p., or as a control, mice were given rat IgG i.p. at 8 h before injection with CneF-CFA, HKC-CFA, or saline-CFA. Three days after immunization, two sponges were implanted in the backs of each mouse, and 4 days later one sponge was injected with saline and the other sponge was injected with CneF. Twenty-four hours after sponge injection, sponges were removed, fluid was collected, and single-cell suspensions were made. (A) Total number of cells determined by hemocytometer counts. (B) Number of activated CD4+ (CD4+ CD45RBlow) T cells as determined by flow cytometry. (C) Level of IFN-γ as shown by ELISA run on the fluid from the sponges. Error bars represent SEM. NS indicates no significant difference.
DISCUSSION
TNF-α is a proinflammatory cytokine necessary for induction of a protective response during a cryptococcal infection (15). Here we show that TNF-α is also necessary for induction of the protective anticryptococcal CMI response induced by the soluble cryptococcal antigen, CneF, in CFA. Furthermore, we have demonstrated that the mechanism by which TNF-α affects the protective anticryptococcal CMI responses is via the accumulation of DC in the lymph nodes draining the site of immunization with the protective immunogen. All three of the DC subsets—which are Langerhans cells, MDC, and LDC—are influenced by TNF-α in our protection model. Thus, the effects of TNF-α on DC in the DLN are quantitative rather than qualitative. It is also clear from our data that activated CD4+-T-cell numbers do not increase in the DLN when DC do not accumulate in the DLN due to neutralization of TNF-α. Induction of a CMI response is a cascade of events including cell-cytokine and cell-cell interactions, resulting in production of activated T cells that recognized the cognate antigen (16). When a cytokine such as TNF-α, which is a required element early in the CMI cascade, is eliminated, the impact on all downstream events can be observed. We have demonstrated this is indeed the case in the cascade of events leading to the induction and expression of the protective anticryptococcal CMI response. Neutralization of TNF-α at the time of immunization not only blocked accumulation of DC in DLN at 18 h after immunization, but also eliminated the increased production of activated CD4+ T cells in the DLN. In accord with this concept, Herring et al. (13) showed that neutralization of TNF-α at the time of infecting mice with C. neoformans via the lungs resulted in diminished levels of TNF-α, interleukin-12, and IFN-γ in lungs. The same group of investigators had previously shown that neutralization of TNF-α at the time of infection reduced the acquired protective response (15). Considering interleukin-12 and IFN-γ are needed for the induction of activated CD4+ Th1 cells and those two cytokines are downstream from accumulation of DC in the cascade of events (16), the combined data from Huffnagle's group fit the consensus scheme of Th1 induction (13, 15). Based on the fact that activated CD4+ T cells were not made in the DLN after neutralization of TNF-α, one would predict that there would be few if any activated CD4+ T cells in circulation in the bloodstream to initiate a DTH reaction. Our findings support these predictions. When TNF-α was neutralized at the time of immunization with CneF-CFA, there was little accumulation of activated CD4+ T cells at the DTH reaction site; the level of IFN-γ, a cytokine produced by activated CD4+ T cells, was near control levels; and weak DTH responses to cryptococcal antigen were observed. When TNF-α was neutralized during the expression phase of the anticryptococcal CMI response, the anticryptococcal DTH response was unaffected. Taken together, our data indicate that TNF-α is essential during induction but not expression of the protective anticryptococcal CMI response, and TNF-α functions by supporting the accumulation of DC, the predominant antigen-presenting cells, in the DLN. Without TNF-α at this very early step in acquisition of an anticryptococcal CMI response, all down-stream events in the response are altered.
From the data we have acquired thus far, we cannot determine whether TNF-α is directly or indirectly causing accumulation of DC in lymph nodes draining the CneF-CFA immunization site. Furthermore, we do not know if the action on DC is in the tissues containing antigen or in the lymph nodes. Based on what is known about DC and TNF-α (7, 26), we speculate that TNF-α is produced in the tissue due to stimulation with CneF. After immature DC in the subcutaneous tissue take up the CneF-CFA, the immature DC are stimulated by TNF-α binding to TNF receptor I (TNFR-I) and/or TNFR-II to mature and to migrate into the regional lymph nodes. We predict that CneF injection into the subcutaneous tissue induces local TNF-α production, because when CneF is injected into gelatin sponges implanted into the backs of mice, TNF-α is produced in the sponges within 6 h after injection (3). It is also possible that some TNF-α production is induced by the CFA. In vitro studies have shown that human monocyte-derived DC are activated to a high level of T-cell-stimulating activity by TNF-α (11), so it is possible that TNF-α has a similar effect on murine DC. If these predictions are correct, then TNF-α would most likely be mediating its effect on the subset(s) of DC expressing TNFR near the injection site of the immunogen. Even though the scenario just discussed seems the most feasible, there are numerous possibilities as to precisely how TNF-α is having its effect on accumulation of the DC in our model, so further studies must be done to address the mode of action of TNF-α on the various DC subsets that accumulate in DLN after immunization with CneF-CFA.
Although TNF-α has a major impact on cells and cytokines either directly or indirectly in the cascade leading to the protective anticryptococcal CMI response, TNF-α does not have a measurable effect on cellularity and DC accumulation in DLN after immunization with the nonprotective cryptococcal immunogen. We have previously shown that after immunization with HKC-CFA, the nonprotective cryptococcal immunogen, DLN cellularity does not change significantly from the control (1). DC and activated CD4+ T cells do not accumulate in the DLN in mice immunized with HKC-CFA (1), so the fact that neutralization of TNF-α had no measurable effect on cellularity and accumulation of DC and activated CD4+ T cells in the DLN in this situation is not surprising. We did observe a significant reduction in footpad swelling after neutralization of TNF-α at the time of immunization with HKC-CFA, but not after neutralization of TNF-α at the time of expression of this response. These findings suggest that TNF-α does play a role in the induction but not the expression phase of the footpad swelling response induced by immunization with HKC-CFA. If the effect of TNF-α is on DC populations or induction of CD4+ T cells in the DLN, it is not a measurable effect. It should be noted that the delayed footpad swelling in response to cryptococcal antigen in mice immunized with HKC-CFA is not a classical DTH response with a mononuclear cell infiltration but rather is a response that is comprised of infiltration of polymorphonuclear lymphocytes (25). The cascade of cellular and cytokine events leading to this nonprotective acquired response to cryptococcal antigen has not been fully defined, but here we show TNF-α has a role during the induction phase of the cascade of events leading to the delayed footpad swelling.
In the experiments in this study, we were able to detect some effects of TNF-α neutralization in mice treated with saline-CFA, and these findings need some explanation. Neutralization of TNF-α significantly reduces the cellularity of the DLN and significantly reduces accumulation of Langerhans cells and LDC in lymph nodes draining the site of saline-CFA injection. Although MDC numbers are reduced also when TNF-α is neutralized, the difference from the control level was not significant at the 95% confidence level. Immunization with saline-CFA induces a classical CMI response to Mycobacterium spp. that can be detected by footpad testing with PPD, a mycobacterial antigen (22). Although we did not evaluate the anti-Mycobacterium DTH response in this study, one can see from our results that TNF-α has a role in the anti-Mycobacterium CMI response similar to that in the induction of the protective anticryptococcal CMI response. Absence of a DTH response to CneF in the saline-CFA-treated mice demonstrates the specificity of the CneF for detection of the anticryptococcal CMI response.
In summary, using an in vivo cryptococcal immunization model, we have shown that TNF-α is necessary for accumulation of Langerhans cells, MDC, and LDC in the DLN after immunization with a cryptococcal antigen that will induce a classical CMI response and protection against a challenge with C. neoformans (23). Neutralization of TNF-α prevents accumulation of these antigen-presenting cells in the DLN, and this results in little to no induction of activated CD4+ T cells that trigger the anticryptococcal DTH response and that are responsible for IFN-γ being at the DTH reaction site. Induction by HKC-CFA of the nonprotective anticryptococcal acquired response, which is detected by delayed footpad swelling to the cryptococcal antigen, is affected by TNF-α, but the mechanism does not appear to be through DC accumulation in DLN as we observe in the case of immunization with the protective cryptococcal immunogen.
Acknowledgments
We greatly appreciate the technical assistance of Kasie L. Nichols and Fredda Schafer. We acknowledge the Flow Cytometry and Cell Sorting Core Facility for Molecular Medicine and St. Francis Research Institute, University of Oklahoma Health Sciences Center, where flow cytometric analyses were performed.
This work was supported in part by National Institutes of Health grants AI-15716, HL-59852, and T32 AI-07364-07.
Editor: T. R. Kozel
REFERENCES
- 1.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan, K. L., and J. W. Murphy. 1993. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 61:2854-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan, K. L., and J. W. Murphy. 1997. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology 90:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan, K. L., and J. W. Murphy. 1994. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 62:2930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington D.C.
- 6.Cauley, L. K., and J. W. Murphy. 1979. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect. Immun. 23:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cumberbatch, M., R. J. Dearman, and I. Kimber. 1997. Langerhans cells require signals from both tumor necrosis factor-α and interleukin-1β for migration. Immunology 92:388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, Z. M., and J. W. Murphy. 1995. Intravascular cryptococcal culture filtrate (CneF) and its major component glucuronoxylomannan (GXM) are potent inhibitors of leukocyte accumulation. Infect. Immun. 63:770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle, H. A., and J. W. Murphy. 1997. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J. Leukoc. Biol. 61:147-155. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, H. A., and J. W. Murphy. 1999. Role of the C-C chemokine, TCA3, in the protective anticryptococcal cell-mediated immune response. J. Immunol. 162:4824-4833. [PubMed] [Google Scholar]
- 11.Geissmann, F., P. Revy, A. Regnault, Y. Lepelletier, M. Dy, N. Brousse, S. H. Amigorena, O., and A. Durandy. 1999. TGF-beta1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 162:4567-4575. [PubMed] [Google Scholar]
- 12.Graybill, J. R., L. Mitchell, and D. J. Drutz. 1979. Host defense in cryptococcosis. III. Protection of nude mice by thymus transplantation. J. Infect. Dis. 140:546-552. [DOI] [PubMed] [Google Scholar]
- 13.Herring, A. C., J. Lee, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-α required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 16.Janeway, C. A., P. Travers, M. Walport, and M. J. Sholmchik. 2001. Immunobiology. The immune system in health and disease, 5th ed. Garland Publishing, New York, N.Y.
- 17.Kovacs, J. A., A. A. Kovacs, M. Polis, W. C. Wright, V. J. Gill, C. U. Tuazon, E. P. Gelmann, H. C. Lane, R. Longfield, G. Overturf, A. M. Macher, A. S. Fauci, J. E. Parrillo, J. E. Bennett, and H. Masur. 1985. Cryptococcosis in the acquired immunodeficiency syndrome. Ann. Intern. Med. 103:533-538. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS: 100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, J. W. 1999. Cell-mediated immunity and medically related fungi, p. 593-621. In M. W. Cunningham, and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 20.Murphy, J. W. 1993. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect. Immun. 61:4750-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy, J. W. 1998. Protective cell-mediated immunity against Cryptococcus neoformans. Res. Immunol. 149:373-386. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, J. W., and R. L. Mosley. 1985. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J. Immunol. 134:577-584. [PubMed] [Google Scholar]
- 23.Murphy, J. W., F. Schafer, A. Casadevall, and A. Adesina. 1998. Antigen-induced protective and non-protective cell-mediated immune components against Cryptococcus neoformans. Infect. Immun. 66:2632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muth, S. M., and J. W. Murphy. 1995. Direct anticryptococcal activity of lymphocytes from Cryptococcus neoformans-immunized mice. Infect. Immun. 63:1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols, K. L., S. K. Bauman, F. B. Schafer, and J. W. Murphy. 2002. Differences in components at the delayed-type hypersensitivity (DH) reaction sites in mice immunized with either a protective or a nonprotective immunogen of Cryptococcus neoformans. Infect. Immun. 70:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piguet, P. F., G. E. Grau, C. Hauser, and P. Vassalli. 1991. Tumor necrosis factor is a critical mediator in hapten-induced irritant and contract hypersensitivity reactions. J. Exp. Med. 173:673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salomon, B., J. L. Cohen, C. Masurier, and D. Klatzmann. 1998. Three populations of mouse lymph node DC with different origins and dynamics. J. Immunol. 160:708-717. [PubMed] [Google Scholar]
- 28.Vremec, D., and K. Shortman. 1997. Dendritic cell subtypes in mouse lymphoid organs cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 159:565-573. [PubMed] [Google Scholar]
- 29.Yuan, R. R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]