Abstract
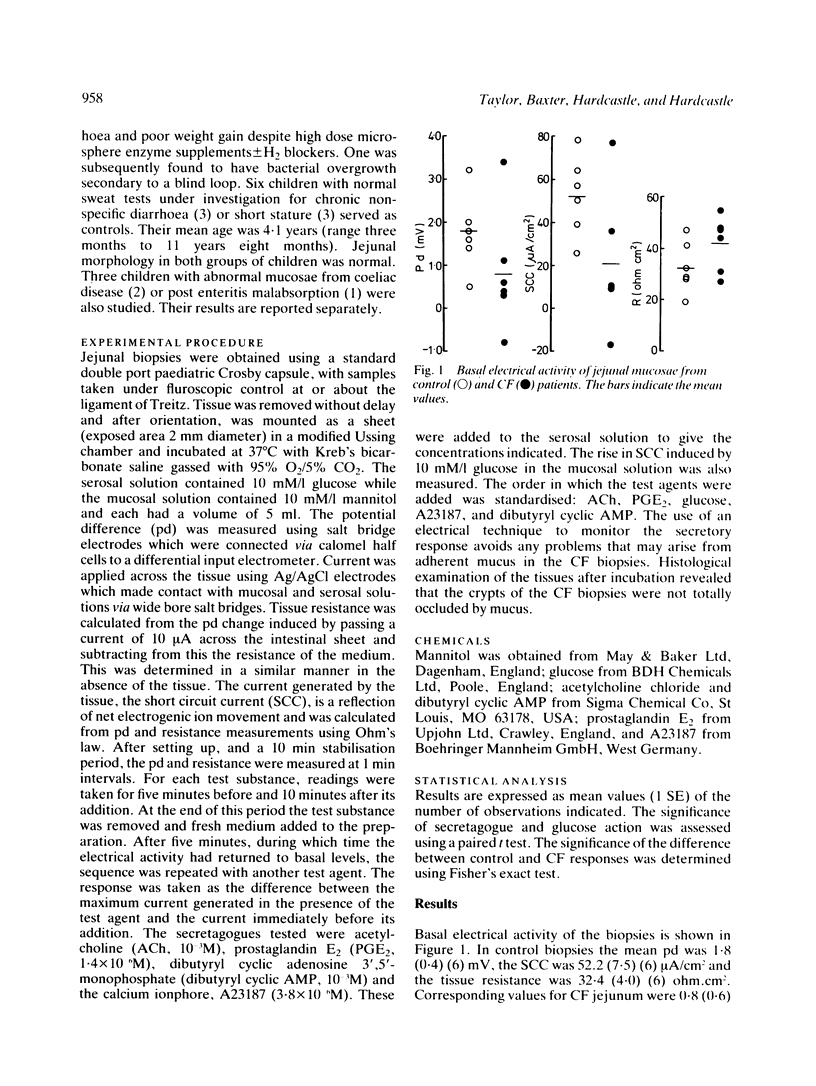
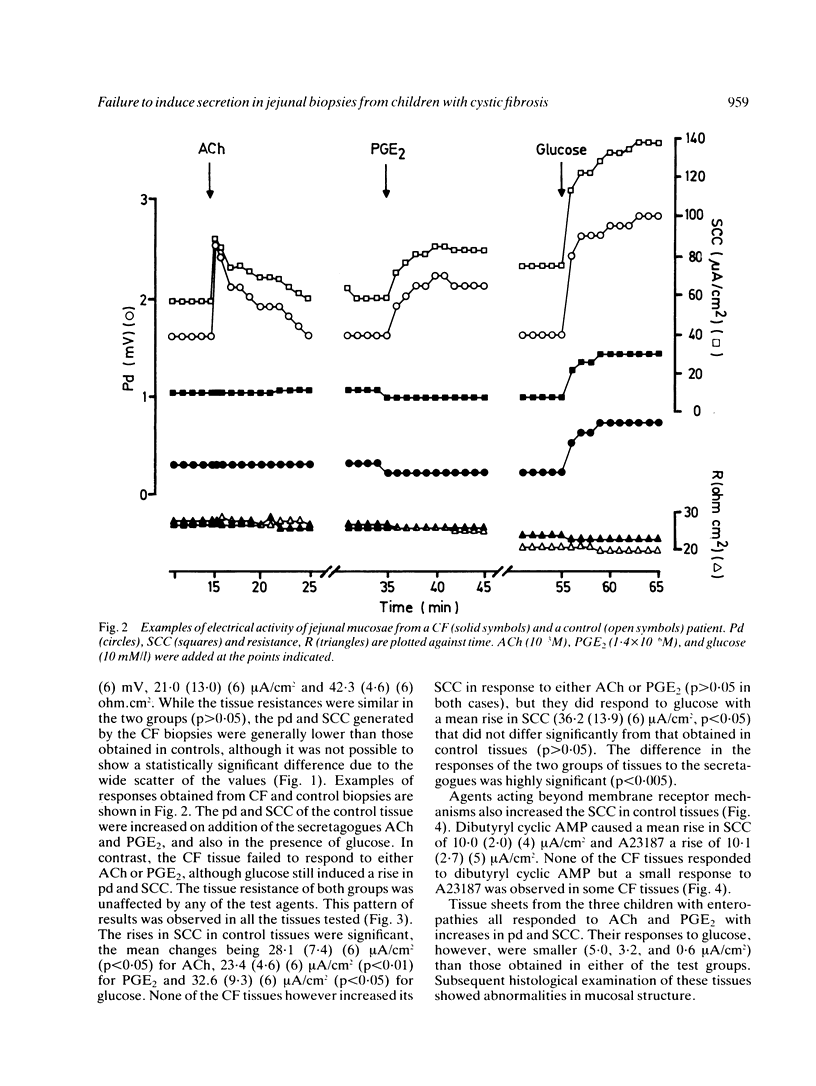
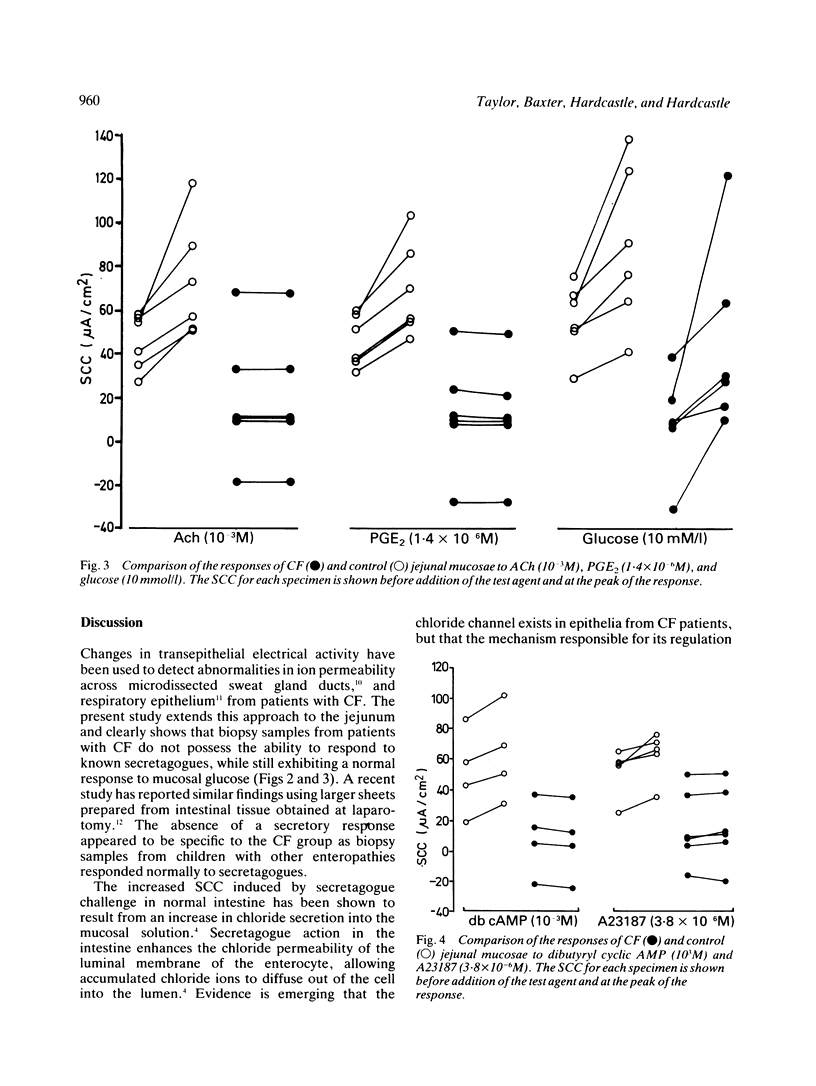
The secretory activity of jejunal biopsies from children with cystic fibrosis (CF) has been investigated using a modified Ussing chamber technique. Samples from six children with CF failed to respond when challenged with the intestinal secretagogues acetylcholine (10(-3) M), prostaglandin E2 (1.4 X 10(-6) M) and dibutyryl cyclic AMP (10(-3) M), while control tissues exhibited rises in short circuit current of 28.1 (7.4) (6) microA/cm2, 23.4 (4.6) (6) microA/cm2 and 10.0 (2.0) (4) microA/cm2 respectively in response to these agents. The calcium ionophore, A23187 (3.8 X 10(-6) M), increased the short circuit current in all the control tissues (mean change = 10.1 (2.7) (5) microA/cm2) and induced a small response in some of the CF tissues. Both groups of tissues generated a rise in short circuit current associated with sodium linked glucose (10 mM/l) absorption (control = 32.6 (9.3) (6) microA/cm2, CF = 36.2 (13.9) (6) microA/cm2, p greater than 0.05). These results show that the defect in chloride transport observed in other epithelia in CF also exists in the jejunum and could contribute to the intestinal effects of the disease. The technique used should permit further studies of the basic defect and may be of diagnostic value.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Boyd C. A. Absence of secretory response in jejunal biopsy samples from children with cystic fibrosis. Lancet. 1987 Aug 15;2(8555):389–389. doi: 10.1016/s0140-6736(87)92402-0. [DOI] [PubMed] [Google Scholar]
- Bukhave K., Rask-Madsen J. Saturation kinetics applied to in vitro effects of low prostaglandin E2 and F 2 alpha concentrations on ion transport across human jejunal mucosa. Gastroenterology. 1980 Jan;78(1):32–42. [PubMed] [Google Scholar]
- Davis B., Shennan D. B., Boyd C. A. Chloride transport in cystic fibrosis placenta. Lancet. 1985 Feb 16;1(8425):392–393. doi: 10.1016/s0140-6736(85)91407-2. [DOI] [PubMed] [Google Scholar]
- Dodge J. A. Gastrointestinal tract and nutrition in cystic fibrosis: pathophysiology. J R Soc Med. 1986;79 (Suppl 12):27–31. [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Giráldez F., Sepúlveda F. V. Changes in the apparent chloride permeability of Necturus enterocytes during the sodium-coupled transport of alanine. Biochim Biophys Acta. 1987 Apr 9;898(2):248–252. doi: 10.1016/0005-2736(87)90044-7. [DOI] [PubMed] [Google Scholar]
- Isaacs P. E., Corbett C. L., Riley A. K., Hawker P. C., Turnberg L. A. In vitro behavior of human intestinal mucosa. The influence of acetyl choline on ion transport. J Clin Invest. 1976 Sep;58(3):535–542. doi: 10.1172/JCI108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Missing Cl conductance in cystic fibrosis. Am J Physiol. 1986 Oct;251(4 Pt 1):C649–C652. doi: 10.1152/ajpcell.1986.251.4.C649. [DOI] [PubMed] [Google Scholar]
- Rohde J. E., Andersen B. In vitro measurement of ion fluxes across biopsies of human jejeunal mucosa during cholera. J Appl Physiol. 1973 Oct;35(4):557–561. doi: 10.1152/jappl.1973.35.4.557. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest. 1984 Jun;73(6):1763–1771. doi: 10.1172/JCI111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Absence of secretory response in jejunal biopsy samples from children with cystic fibrosis. Lancet. 1987 Jul 11;2(8550):107–108. doi: 10.1016/s0140-6736(87)92781-4. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]


