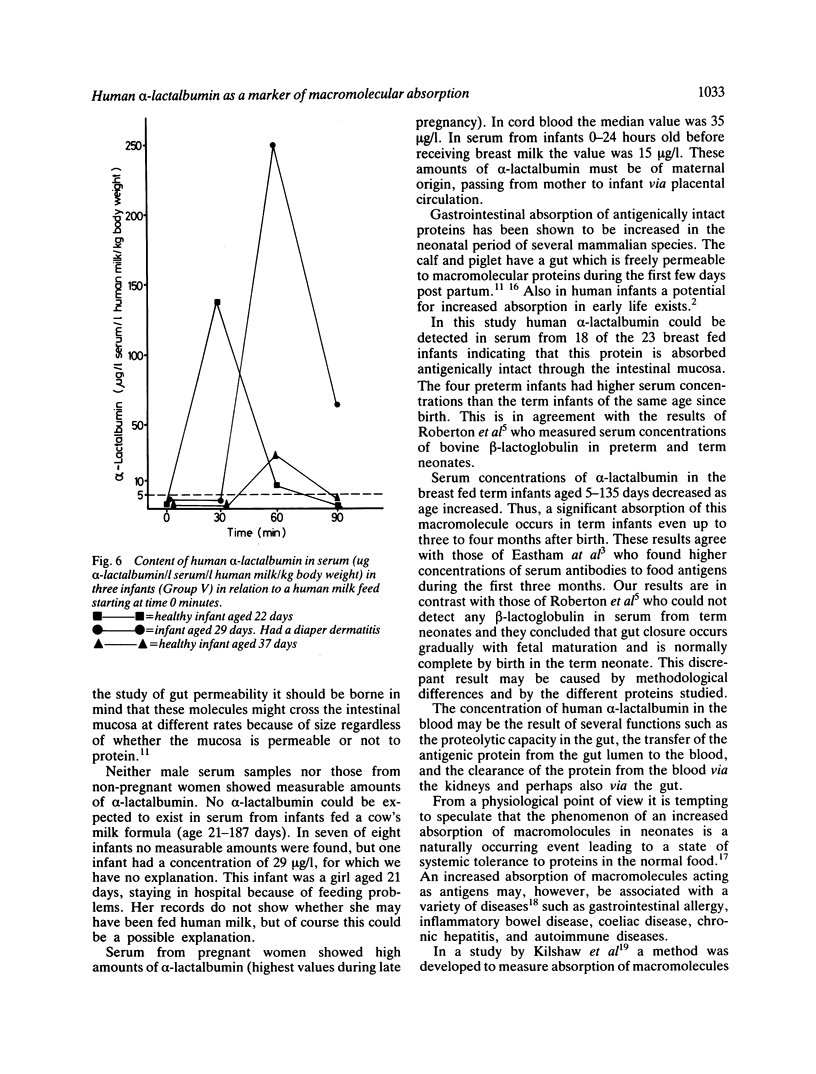
Abstract
alpha-Lactalbumin was purified from human milk and a competitive radioimmunoassay for measuring serum concentrations of human alpha-lactalbumin was developed. Human alpha-lactalbumin was not detected (less than 5 micrograms/l) in serum from adult men (n = 4), non-pregnant women (n = 6) or in serum from seven of eight formula fed infants. alpha-Lactalbumin was found in serum from pregnant women (19-130 micrograms/l, n = 4), cord blood (22-72 micrograms/l, median value 35 micrograms/l, n = 9), and from newborn non-fed infants (less than 1 day old) (less than 5-50 micrograms/l, median value 15 micrograms/l, n = 11). In breast fed infants the serum concentration of alpha-lactalbumin was highest in preterm infants (140-952 micrograms/l serum/l human milk/kg body weight, n = 4) and decreased in term infants successively with maturity (age 5-30 days: median value 85 micrograms/l serum/l human milk/kg body weight, n = 7; age 31-60 days: median value 43, n = 6; age 61-135 days: median value 12, n = 6). A human milk feeding to three infants one month of age gave serum peak values of alpha-lactalbumin after 30 to 60 minutes. We suggest that human alpha-lactalbumin is a suitable marker for investigating macromolecular absorption in physiological and pathological conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach R. C., Menzies I. S., Clayden G. S., Scopes J. W. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child. 1982 Feb;57(2):141–145. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J. The local immune response. Am J Vet Res. 1975 Apr;36(4 Pt 2):488–491. [PubMed] [Google Scholar]
- Dannaeus A., Inganäs M., Johansson S. G., Foucard T. Intestinal uptake of ovalbumin in malabsorption and food allergy in relation to serum IgG antibody and orally administered sodium cromoglycate. Clin Allergy. 1979 May;9(3):263–270. doi: 10.1111/j.1365-2222.1979.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Eastham E. J., Lichauco T., Grady M. I., Walker W. A. Antigenicity of infant formulas: role of immature intestine on protein permeability. J Pediatr. 1978 Oct;93(4):561–564. doi: 10.1016/s0022-3476(78)80888-9. [DOI] [PubMed] [Google Scholar]
- Forsum E. Determination of alpha-lactalbumin in human milk. J Dairy Sci. 1976 Jan;59(1):14–18. doi: 10.3168/jds.S0022-0302(76)84148-3. [DOI] [PubMed] [Google Scholar]
- Fälth-Magnusson K., Kjellman N. I., Magnusson K. E., Sundqvist T. Intestinal permeability in healthy and allergic children before and after sodium-cromoglycate treatment assessed with different-sized polyethyleneglycols (PEG 400 and PEG 1000). Clin Allergy. 1984 May;14(3):277–286. doi: 10.1111/j.1365-2222.1984.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Jackson P. G., Lessof M. H., Baker R. W., Ferrett J., MacDonald D. M. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981 Jun 13;1(8233):1285–1286. doi: 10.1016/s0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- Jakobsson I., Lindberg T., Benediktsson B., Hansson B. G. Dietary bovine beta-lactoglobulin is transferred to human milk. Acta Paediatr Scand. 1985 May;74(3):342–345. doi: 10.1111/j.1651-2227.1985.tb10981.x. [DOI] [PubMed] [Google Scholar]
- Kleinman R. E., Walker W. A. Antigen processing and uptake from the intestinal tract. Clin Rev Allergy. 1984 Feb;2(1):25–37. doi: 10.1007/BF02991209. [DOI] [PubMed] [Google Scholar]
- Lecce J. G. Absorption of macromolecules by neonatal intestine. Biol Neonat. 1965;9(1):50–61. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Roberton D. M., Paganelli R., Dinwiddie R., Levinsky R. J. Milk antigen absorption in the preterm and term neonate. Arch Dis Child. 1982 May;57(5):369–372. doi: 10.1136/adc.57.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Tagesson C., Sjödahl R. Passage of molecules through the wall of the gastrointestinal tract. Urinary recovery of different-sized polyethylene glycols after intravenous and intestinal deposition. Scand J Gastroenterol. 1984 May;19(3):315–320. [PubMed] [Google Scholar]
- Udall J. N., Walker W. A. The physiologic and pathologic basis for the transport of macromolecules across the intestinal tract. J Pediatr Gastroenterol Nutr. 1982;1(3):295–301. doi: 10.1097/00005176-198201030-00004. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Weström B., Svendsen J., Tagesson C. Intestinal permeability to polyethyleneglycol 600 in relation to macromolecular 'closure' in the neonatal pig. Gut. 1984 May;25(5):520–525. doi: 10.1136/gut.25.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]



