Abstract
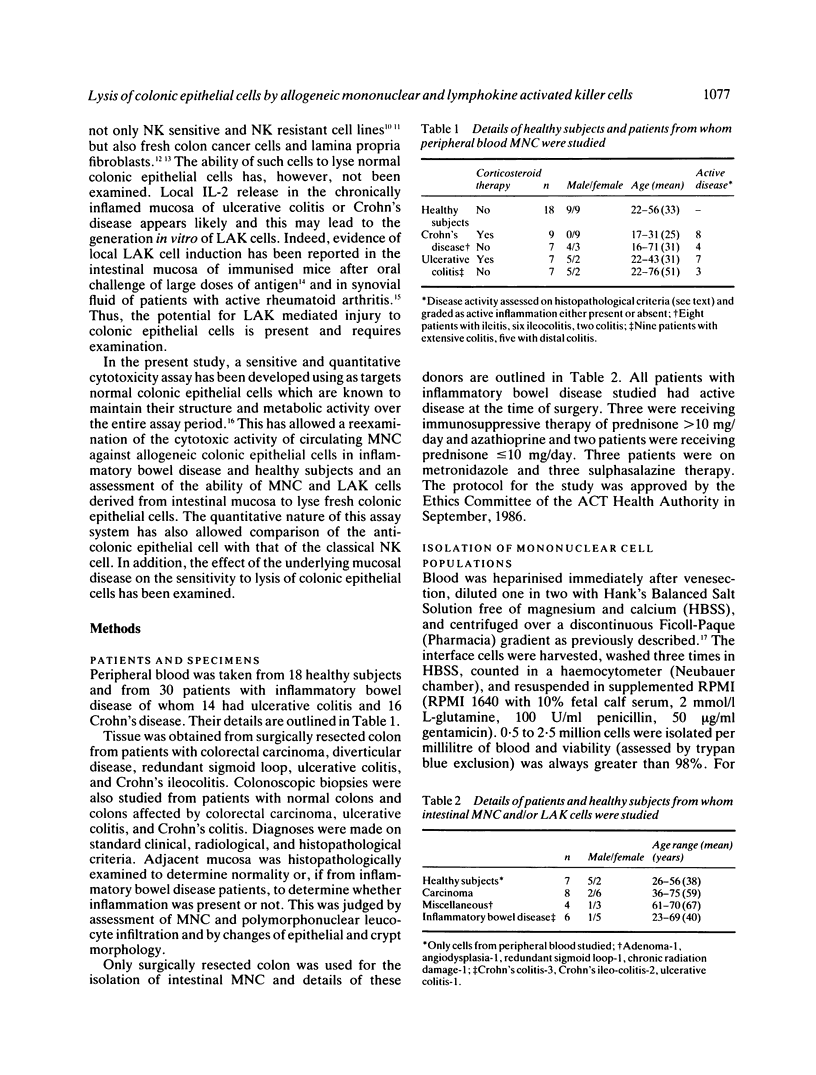
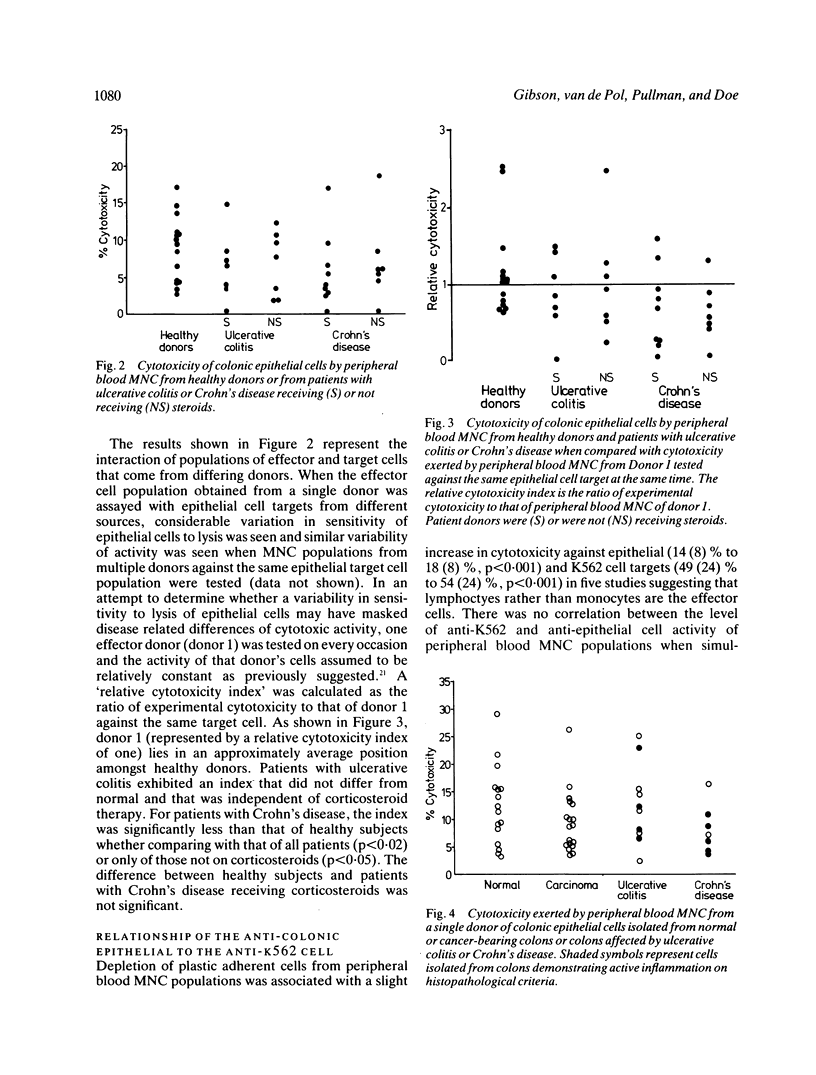
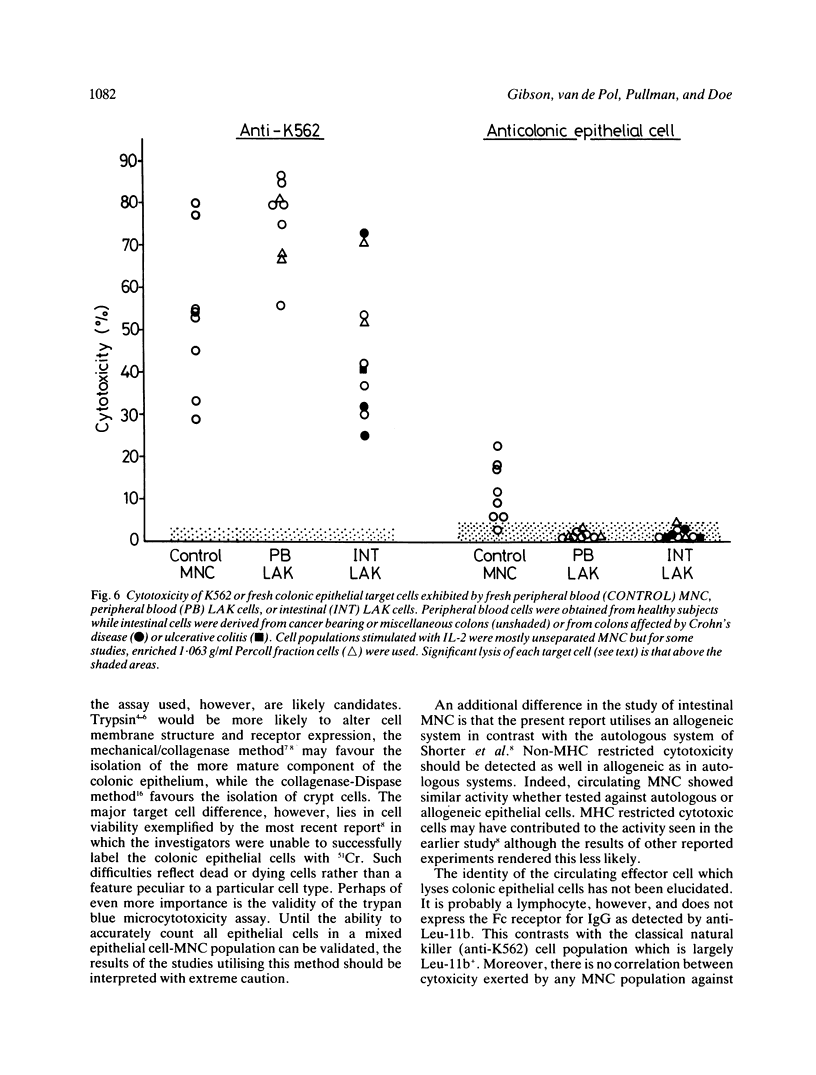
A sensitive 4 h 51Cr-release cytotoxicity assay has been developed using as targets colonic epithelial cells obtained by Dispase-collagenase digestion of resected mucosa or colonoscopic biopsies. Peripheral blood mononuclear cells (MNC) from most healthy donors showed low, but significant levels of cytotoxicity for normal epithelial cell target cells of 8.7 (4.4) % (mean (SD] and similar levels were found in 14 ulcerative colitis (6.5 (4.4) %) and 16 Crohn's disease (6.2 (5.2) %) patients. Neither drug therapy nor disease activity influenced the results. The sensitivity of colonic epithelial cells isolated from inflamed and histologically normal mucosa to lysis by peripheral blood MNC from a single donor was not affected by the underlying disease. Anti-epithelial cell activity did not correlate with anti-K562 activity and the cytotoxic cell was plastic non-adherent and Leu-11b-. None of 15 MNC populations isolated from mucosa of normal, tumour bearing, or chronically inflamed intestine exhibited significant lysis of colonic epithelial cells despite killing of K562 target cells in 10. Lymphokine activated killer (LAK) cells, generated by interleukin-2 stimulation in vitro of nine intestinal and seven peripheral blood MNC populations, exhibited high levels of lysis of K562 cells but, on every occasion, failed to lyse colonic epithelial cells. These data indicate that spontaneously cytotoxic or LAK cells are unlikely to play a role in the generation of colonic epithelial cell injury by direct cytotoxicity in inflammatory bowel disease.
Full text
PDF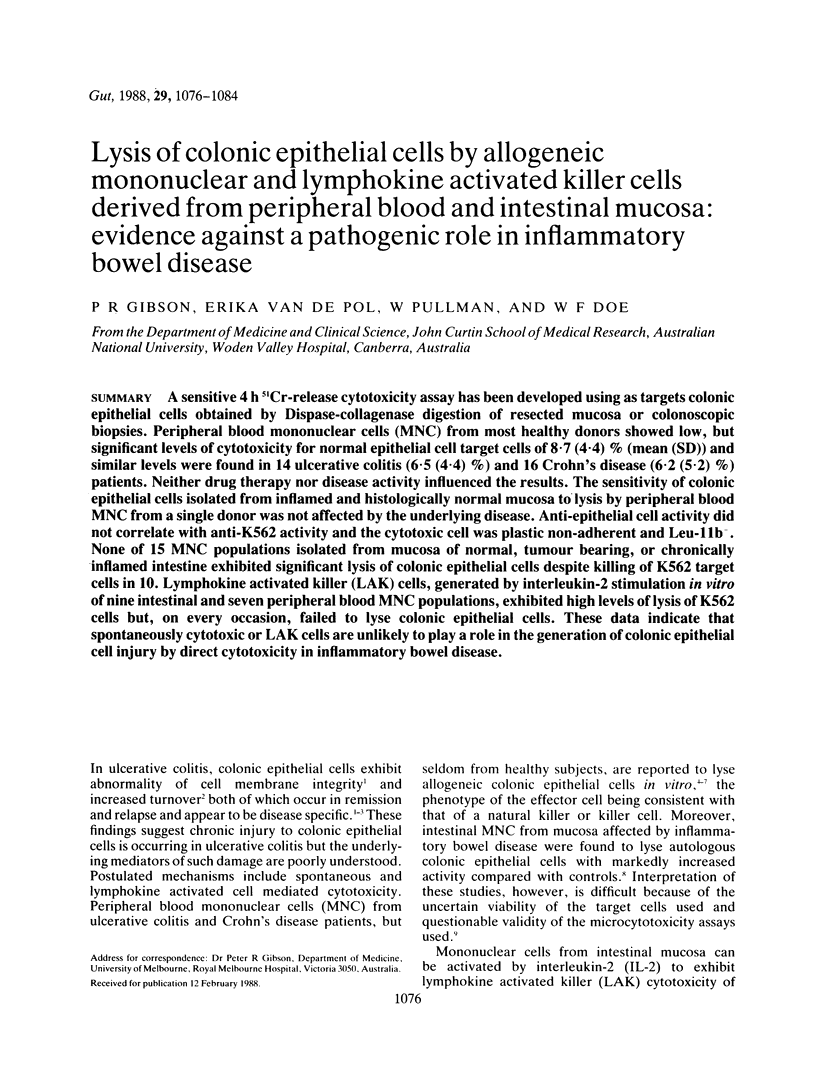





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borberg H., Oettgen H. F., Choudry K., Beattie E. J., Jr Inhibition of established transplants of chemically induced sarcomas in syngeneic mice by lymphocytes from immunized donors. Int J Cancer. 1972 Nov;10(3):539–547. doi: 10.1002/ijc.2910100312. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Chiba M., Bartnik W., ReMine S. G., Thayer W. R., Shorter R. G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut. 1981 Mar;22(3):177–186. doi: 10.1136/gut.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan E. A., Eddleston A. L. Immunotherapy for cancer: the use of lymphokine activated killer (LAK) cells. Gut. 1987 Feb;28(2):113–116. doi: 10.1136/gut.28.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C., Tubbs R. R., Youngman K. R. Human intestinal mucosal mononuclear cells exhibit lymphokine-activated killer cell activity. Gastroenterology. 1985 Mar;88(3):625–637. doi: 10.1016/0016-5085(85)90130-1. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Dow E. L., Selby W. S., Strickland R. G., Jewell D. P. Natural killer cells and spontaneous cell-mediated cytotoxicity in the human intestine. Clin Exp Immunol. 1984 May;56(2):438–444. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Hermanowicz A., Jewell D. P. Factors affecting the spontaneous cell-mediated cytotoxicity of intestinal mononuclear cells. Immunology. 1984 Oct;53(2):267–274. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Hermanowicz A., Verhaar H. J., Ferguson D. J., Bernal A. L., Jewell D. P. Isolation of intestinal mononuclear cells: factors released which affect lymphocyte viability and function. Gut. 1985 Jan;26(1):60–68. doi: 10.1136/gut.26.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Jewell D. P. Local immune mechanisms in inflammatory bowel disease and colorectal carcinoma. Natural killer cells and their activity. Gastroenterology. 1986 Jan;90(1):12–19. doi: 10.1016/0016-5085(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Jewell D. P. The nature of the natural killer (NK) cell of human intestinal mucosa and mesenteric lymph node. Clin Exp Immunol. 1985 Jul;61(1):160–168. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., van de Pol E., Barratt P. J., Doe W. F. Ulcerative colitis--a disease characterised by the abnormal colonic epithelial cell? Gut. 1988 Apr;29(4):516–521. doi: 10.1136/gut.29.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Zvaifler N. J. Characterization of the natural killer-like lymphocytes in rheumatoid synovial fluid. J Immunol. 1985 Mar;134(3):1483–1486. [PubMed] [Google Scholar]
- Hogan P. G., Hapel A. J., Doe W. F. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J Immunol. 1985 Sep;135(3):1731–1738. [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. P., Strober W. Cytotoxic lymphocytes and intestinal disease. Gastroenterology. 1986 Jan;90(1):235–237. doi: 10.1016/0016-5085(86)90098-3. [DOI] [PubMed] [Google Scholar]
- Kemler B. J., Alpert E. Inflammatory bowel disease: study of cell mediated cytotoxicity for isolated human colonic epithelial cells. Gut. 1980 May;21(5):353–359. doi: 10.1136/gut.21.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Kagnoff M. F. Nonspecific recruitment of cytotoxic effector cells in the intestinal mucosa of antigen-primed mice. J Exp Med. 1984 Dec 1;160(6):1931–1936. doi: 10.1084/jem.160.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere R., Rosenberg S. A. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Res. 1985 Aug;45(8):3735–3741. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- MacDermott R. P., Franklin G. O., Jenkins K. M., Kodner I. J., Nash G. S., Weinrieb I. J. Human intestinal mononuclear cells. I. Investigation of antibody-dependent, lectin-induced, and spontaneous cell-mediated cytotoxic capabilities. Gastroenterology. 1980 Jan;78(1):47–56. [PubMed] [Google Scholar]
- PERLMANN P., BROBERGER O. In vitro studies of ulcerative colitis. II. Cytotoxic action of white blood cells from patients on human fetal colon cells. J Exp Med. 1963 May 1;117:717–733. doi: 10.1084/jem.117.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Roediger W. E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980 Oct 4;2(8197):712–715. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Shorter R. G., Cardoza M., Spencer R. J., Huizenga K. A. Further studies on in vitro cytotoxicity of lymphocytes from patients with ulcerative and granulomatous colitis for allogeneic colonic epithelial cells, including the effects of colectomy. Gastroenterology. 1969 Feb;56(2):304–309. [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Smith H. G., Harmel R. P., Hanna M. G., Jr, Zwilling B. S., Zbar B., Rapp H. J. Regression of established intradermal tumors and lymph node metastases in guinea pigs after systemic transfer of immune lymphoid cells. J Natl Cancer Inst. 1977 May;58(5):1315–1322. doi: 10.1093/jnci/58.5.1315. [DOI] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. A method for the production and quantitative assay of human lymphokine preparations. J Immunol Methods. 1981;41(1):9–21. doi: 10.1016/0022-1759(81)90269-6. [DOI] [PubMed] [Google Scholar]
- Watson D. W., Quigley A., Bolt R. J. Effect of lymphocytes from patients with ulcerative colitis on human adult colon epithelial cells. Gastroenterology. 1966 Dec;51(6):985–993. [PubMed] [Google Scholar]



