Abstract
The prevalence of deoxyribonucleic acid (DNA) aneuploidy in 297 samples from 38 patients with ulcerative colitis of varying duration was investigated by flow cytometry. In 12 patients colitis was complicated by the development of colorectal carcinoma: one had three synchronous carcinomas. Only four of 14 carcinomas were DNA aneuploid. Deoxyribonucleic acid aneuploidy occurred focally in the colorectal mucosa in the presence and absence of carcinoma: rates of aneuploidy (67% in cancer patients and 42% in non-cancer patients), were not significantly different (chi 2 = 1.0962, p = 0.295). A higher rate of DNA aneuploidy was found in dysplastic tissues (21%) compared with non-dysplastic tissues (15%), but again these differences did not reach statistical significance (chi 2 = 1.0747, p = 0.299). Deoxyribonucleic acid aneuploidy and dysplastic change occurred more often with increasing duration of ulcerative colitis (p less than 0.001, p less than 0.005 respectively). We conclude that flow cytometric analysis of cellular DNA content should not replace present morphological methods of assessment of premalignancy in ulcerative colitis, but may be a useful adjunct in the identification of abnormal mucosa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. C., Biggart J. D., Orchin J. C., Foster H. An immunoperoxidase study of epithelial marker antigens in ulcerative colitis with dysplasia and carcinoma. J Clin Pathol. 1985 Jan;38(1):18–29. doi: 10.1136/jcp.38.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage N. C., Robins R. A., Evans D. F., Turner D. R., Baldwin R. W., Hardcastle J. D. The influence of tumour cell DNA abnormalities on survival in colorectal cancer. Br J Surg. 1985 Oct;72(10):828–830. doi: 10.1002/bjs.1800721018. [DOI] [PubMed] [Google Scholar]
- Blackstone M. O., Riddell R. H., Rogers B. H., Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981 Feb;80(2):366–374. [PubMed] [Google Scholar]
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dombal F. T., Watts J. M., Watkinson G., Goligher J. C. Local complications of ulcerative colitis: stricture, pseudopolyposis, and carcinoma of colon and rectum. Br Med J. 1966 Jun 11;1(5501):1442–1447. doi: 10.1136/bmj.1.5501.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Naunton Morgan M., Filipe M. I., Gazzard B. Sialomucins in the assessment of dysplasia and cancer-risk patients with ulcerative colitis treated with colectomy and ileo-rectal anastomosis. Histopathology. 1985 Feb;9(2):223–235. doi: 10.1111/j.1365-2559.1985.tb02437.x. [DOI] [PubMed] [Google Scholar]
- Hammarberg C., Slezak P., Tribukait B. Early detection of malignancy in ulcerative colitis. A flow-cytometric DNA study. Cancer. 1984 Jan 15;53(2):291–295. doi: 10.1002/1097-0142(19840115)53:2<291::aid-cncr2820530218>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W. Application of DNA flow cytometry to paraffin-embedded archival material for the study of aneuploidy and its clinical significance. Cytometry. 1985 Jul;6(4):327–333. doi: 10.1002/cyto.990060409. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hendriksen C., Kreiner S., Binder V. Long term prognosis in ulcerative colitis--based on results from a regional patient group from the county of Copenhagen. Gut. 1985 Feb;26(2):158–163. doi: 10.1136/gut.26.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewenter J., Ahlman H., Hultén L. Cancer risk in extensive ulcerative colitis. Ann Surg. 1978 Dec;188(6):824–828. doi: 10.1097/00000658-197812000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard-Jones J. E., Morson B. C., Ritchie J. K., Shove D. C., Williams C. B. Cancer in colitis: assessment of the individual risk by clinical and histological criteria. Gastroenterology. 1977 Dec;73(6):1280–1289. [PubMed] [Google Scholar]
- Lennard-Jones J. E., Morson B. C., Ritchie J. K., Williams C. B. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet. 1983 Jul 16;2(8342):149–152. doi: 10.1016/s0140-6736(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Quirke P., Fozard J. B., Dixon M. F., Dyson J. E., Giles G. R., Bird C. C. DNA aneuploidy in colorectal adenomas. Br J Cancer. 1986 Apr;53(4):477–481. doi: 10.1038/bjc.1986.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Shields H. M., Bates M. L., Goldman H., Zuckerman G. R., Mills B. A., Best C. J., Bair F. A., Goran D. A., DeSchryver-Kecskemeti K. Scanning electron microscopic appearance of chronic ulcerative colitis with and without dysplasia. Gastroenterology. 1985 Jul;89(1):62–72. doi: 10.1016/0016-5085(85)90746-2. [DOI] [PubMed] [Google Scholar]
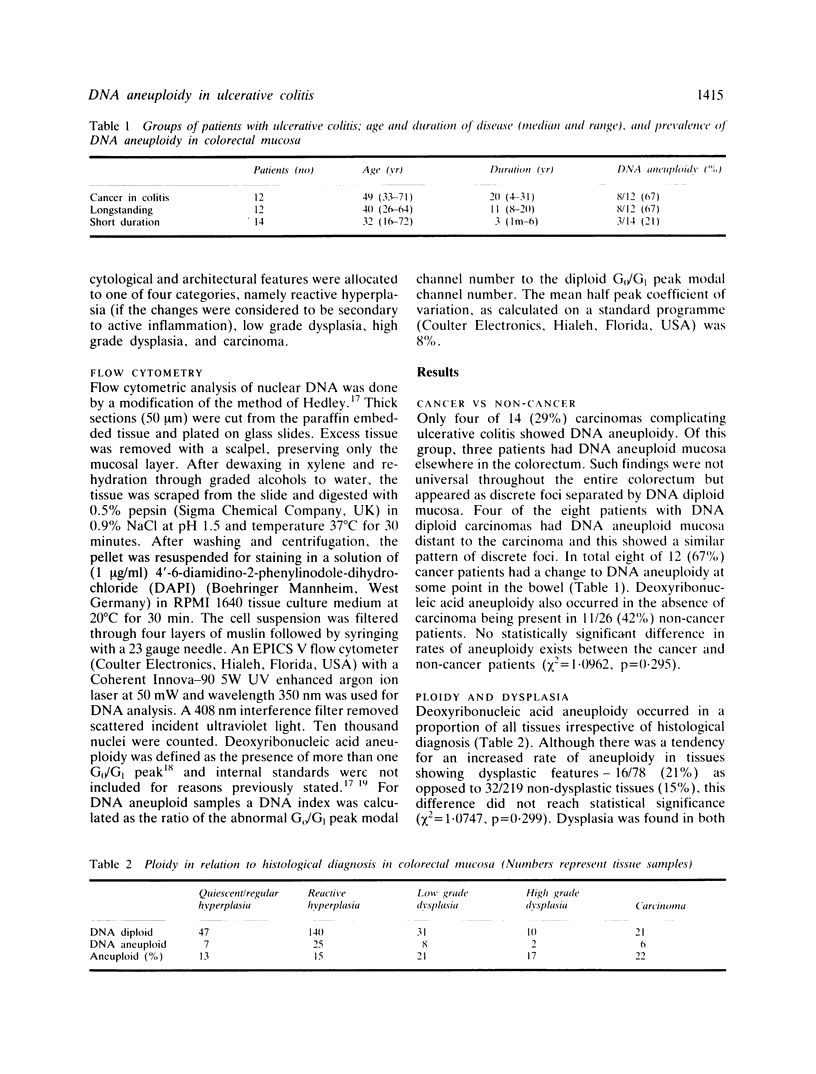
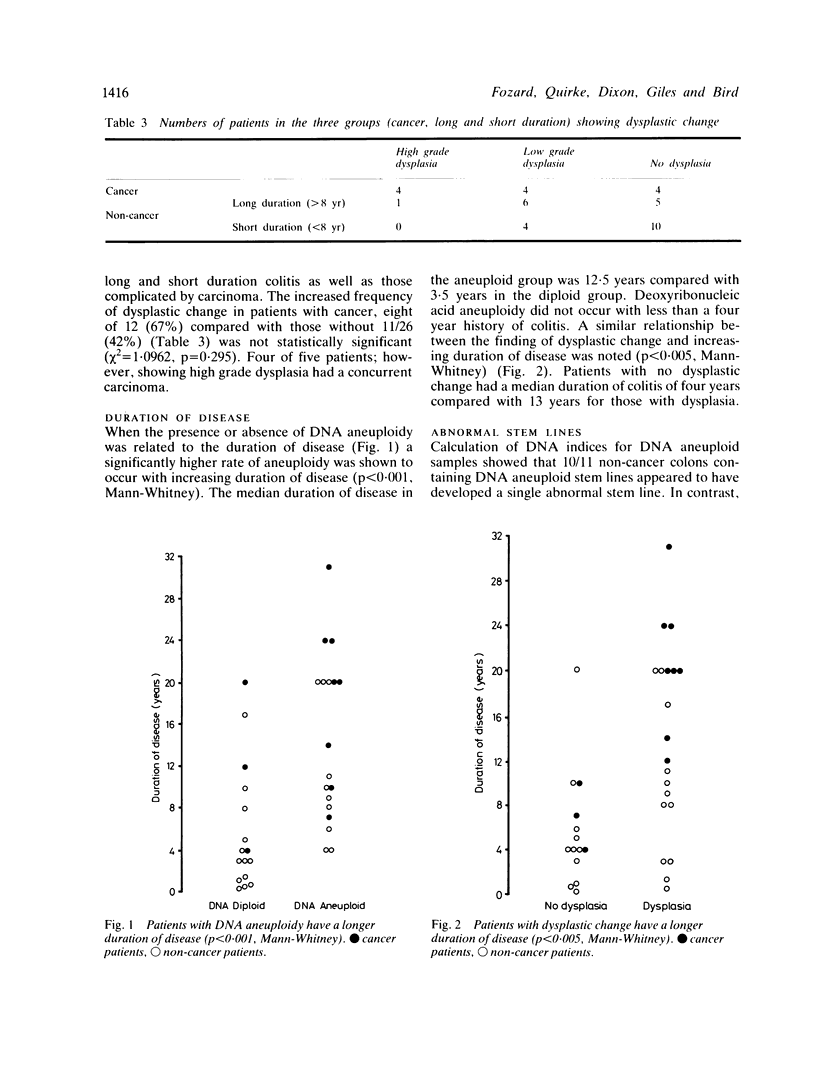
- Tribukait B., Hammarberg C., Rubio C. Ploidy and proliferation patterns in colo-rectal adenocarcinomas related to Dukes' classification and to histopathological differentiation. A flow-cytometric DNA study. Acta Pathol Microbiol Immunol Scand A. 1983 Mar;91(2):89–95. [PubMed] [Google Scholar]
- van den Ingh H. F., Griffioen G., Cornelisse C. J. Flow cytometric detection of aneuploidy in colorectal adenomas. Cancer Res. 1985 Jul;45(7):3392–3397. [PubMed] [Google Scholar]


