Abstract
This study expanded our earlier finding that Shiga toxin type 1 (Stx1) has activity against bovine leukemia virus (BLV) (W. A. Ferens and C. J. Hovde, Infect. Immun. 68:4462-4469, 2000). The Stx molecular motifs required for antiviral activity were identified, and a mechanism of Stx action on virally infected cells is suggested. Using inhibition of BLV-dependent spontaneous lymphocyte proliferation as a measure of antiviral activity, we showed that Stx2 had antiviral activity similar to that of Stx1. Enzymatic and antiviral activities of three StxA1 chain mutants deficient in enzymatic activity or aspects of receptor-mediated cytotoxicity were compared. Using protein synthesis inhibition to measure enzymatic activity, the mutant E167D was 300-fold less catalytically active than wild-type StxA1, was minimally active in antiviral assays, and did not inhibit synthesis of viral proteins. Two StxA1 mutants, A231D-G234E and StxA11 (enzymatically active but unable to kill cells via the classical receptor-mediated route), had undiminished antiviral activity. Although binding of radiolabeled StxA1 to bovine blood cells or to free virus was not detected, flow cytometric analysis showed that the number of BLV-expressing cells were specifically reduced in cultures treated with Stx. These unique and rare lymphocytes were highly permeable to 40- and 70-kDa fluorescent dextrans, indicating that direct absorption of toxins by virus-expressing cells is a potential mechanism of target cell intoxication. These results support the hypothesis that Stx-producing Escherichia coli colonization of the gastrointestinal tract may benefit ruminant hosts by the ability of Stxs to exert antiviral activity.
Ruminant animals are a reservoir for Shiga toxin (Stx)-producing Escherichia coli (STEC) that can cause hemorrhagic colitis and life-threatening sequelae in humans (15, 23, 25). STEC are part of the normal ruminant gastrointestinal microbiota and are frequently isolated from cattle, sheep, and deer (8, 38, 41, 45). Surveys of healthy domesticated cattle routinely show a high prevalence of STEC in animals around the world (7, 8, 10, 12, 34-36). The reasons for the wide distribution of STEC in ruminants are not known. The possible benefits arising from gastrointestinal tract colonization by STEC include postulated enhancement of gastrointestinal mucosal architecture (E. D. E. Hoey, C. G. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. E. Smith, Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001, Abstr. B-222, 2001) and antiviral activity (17).
Bovine leukemia virus (BLV) is an oncogenic retrovirus that infects B lymphocytes and induces chronic, benign, mostly subclinical infection in cattle (20). Most BLV-infected animals experience some elevation in peripheral B lymphocyte numbers, and about one-third develop persistent lymphocytosis, a preneoplastic polyclonal expansion of peripheral B lymphocytes containing provirus (16). The majority of these cattle remain clinically normal, but a small percentage develop a B-cell lymphosarcoma (21). Although resistance to BLV disease includes humoral and cellular immunity (21), the presence of STEC flora may constitute another factor limiting the viremia and disease progression. A hallmark of peripheral blood mononuclear cells (PBMC) from cattle in the persistently lymphocytotic stage of BLV infection is spontaneous lymphocyte proliferation (SLP) when placed in culture (55, 56). SLP is initiated by a rapid derepression of viral gene transcription and viral protein synthesis (3, 20, 21), so it is a measure of viral activity. Previous work by Ferens and Hovde shows that Stx specifically blocks BLV-dependent initiation of SLP and does not cause indiscriminate cell death (17). On this basis, we used cultured PBMC from BLV-positive cattle as a model system for investigating the antiviral activity of the Stxs. Although inhibition of SLP is not a direct measure of antiviral activity, we refer to it as an antiviral assay rather than as a cell proliferation inhibition assay because it represents cell division specifically triggered by BLV derepression.
The family of Stxs includes Stx1, Stx2, and Stx2 variants (13). These toxins belong to a larger group of proteins called the ribosome-inactivating proteins (RIPs) (found in a variety of higher plants and some bacteria) that share structural features and have N-glycosidase enzymatic activity. Intoxication of eukaryotic cells by RIPs leads to a rapid inhibition of protein synthesis and cell death (49). Most RIPs are hemitoxins (enzymatically active A chains), and some are holotoxins (one A chain associated with a specific number of B chains). B subunits mediate toxin binding to receptors on eukaryotic cells and receptor-mediated endocytosis (31). Thus, holotoxins are highly toxic to cells expressing receptors for a B subunit(s), but not to receptor-deprived cells, and are not toxic to normal cells as isolated A chains (5, 14, 24). Plant hemitoxins are not toxic to plants that synthesize them and have low cytotoxicity against animal cells, unless the cells have high pinocytic activity (11, 59). However, these hemitoxins can enter and eliminate virally infected plant cells (4) and some are also found to be highly toxic to various virally infected animal cells (24).
Stxs have a single enzymatically active ∼32-kDa A subunit noncovalently associated with a pentamer of ∼7.7-kDa B subunits. The A subunit is an N-glycosidase that cleaves a specific adenine residue on 28S rRNA in 60S ribosomal subunits (13, 26), and the pentamer of B subunits mediates binding of holotoxin to toxin receptors. Stx1 and Stx2 bind to globotriosylceramide (Gb3) (31, 33) expressed by Vero cells and also by other types of sensitive cells, including human renal endothelial cells (42). Following internalization, toxin enters the cytosol via retrograde transport from the trans-Golgi network (47). The A chain is proteolytically cleaved into a 27.5-kDa A1 fragment (enzymatically active) and a small A2 fragment that, in an intact A chain, obstructs access to the catalytic center and mediates A-B association (2, 22). Thus, receptor-mediated cytotoxicity of Stxs requires an enzymatically active A chain capable of association with B subunits and able to complete retrograde transport into the cytosol. Consequently, StxA1 chain mutations that abolish receptor-mediated Stx1 cytotoxicity include mutations in the catalytic center, mutations that render the A chain unable to associate with B subunits, or mutations within a hydrophobic region of StxA1 required for cell trafficking. Thus, we selected three previously constructed and characterized StxA1 mutants, each one deficient in a different aspect of receptor-mediated cytotoxicity, for analysis of antiviral activity. The enzymatic mutant E167D has several-hundred-fold-lower enzymatic activity than wild-type StxA1 due to a replacement, within the catalytic center, of a glutamic acid 167 with an aspartic acid (28). The cell-trafficking mutant, A231D-G234E, is enzymatically active but crippled in retrograde transport due to replacements (outside of the catalytic center) of an alanine 231 with an aspartic acid and a glycine 234 with a glutamic acid (54). The association mutant StxA11 is enzymatically active but unable to associate with B subunits due to a deletion of 39 amino acids at the carboxy terminus (2).
Here we expanded the initial finding by Ferens and Hovde of Stx1 antiviral activity (17) by (i) measurement of Stx2 antiviral activity; (ii) analysis of antiviral activity of three specific mutant toxins, each deficient in different molecular motifs required for receptor-mediated cytotoxicity; (iii) characterization of the identity of the presumptive cellular targets; (iv) assessment of StxA1 binding to bovine cells and to viral particles; and (v) exploration of the permeability of target cells.
MATERIALS AND METHODS
Animals.
Holstein cows naturally infected with BLV were from the dairy herd of the University of Idaho (Moscow, Idaho). These BLV-positive cattle were seropositive for antibody to the BLV protein gp51 by agar gel immunodiffusion and were in an advanced (persistently lymphocytotic) subclinical stage of disease (6). BLV-negative cattle were from the BLV-free herd at Washington State University Knotts Dairy Center (Pullman, Wash.), were seronegative for BLV, and maintained normal white-blood-cell counts.
Toxins.
Wild-type StxA1 and mutants of StxA1 were purified using previously described methods (28, 54, 60). Briefly, periplasmic proteins were extracted from recombinant E. coli by treatment with polymyxin B sulfate (50 μg/ml), concentrated by 80% ammonium sulfate precipitation, dialyzed, and adsorbed to a Matrex Gel Green A agarose column (Amicon, Beverly, Mass.) equilibrated with 10 mM phosphate-buffered saline (PBS). The toxins were eluted at ∼0.3 M NaCl by using a gradient of 0.15 to 1.0 M NaCl. Toxins were dialyzed against 10 mM PBS, and their concentrations were measured using a Bio-Rad microassay with bovine serum albumin as a standard. Wild-type StxA1 was purified from E. coli SY327(pSC25) (28), the enzymatic mutant E167D was purified from E. coli SY327(pSC25.1) (28), the cell-trafficking mutant A231D-G234E was purified from E. coli SY327(pUCΔH25) (54), and the A-B association mutant StxA11 was purified from E. coli DH5α(pRD500) (2). Wild-type Stx2 holotoxin was a gift from A. D. O'Brien (Uniformed Services University of the Health Sciences, Bethesda, Md.) (37).
Protein synthesis inhibition assay.
Enzymatic activity of the toxins was measured in a protein synthesis inhibition assay by using a luciferase assay system (Promega Corporation, Madison, Wis.) according to the manufacturer's instructions. Briefly, various amounts of purified toxins in PBS were preincubated with rabbit reticulocyte lysate at 30°C for 20 min. Following preincubation, the lysates were combined with leucine-free and methionine-free amino acid mix, RNasin RNase inhibitor, luciferase RNA, and nuclease-free water. Protein synthesis was allowed to proceed for 90 min, an aliquot of the reaction mixture was combined with luciferase assay reagent, and the resulting chemiluminescence was measured over a 10-s period with a 2-s delay, using a luminometer (Lumat LB 9507; Berthold Technologies U.S.A., Oak Ridge, Tenn.).
Lymphocyte culture and spontaneous proliferation assay.
Blood was collected from cows into acid citrate dextrose (1 part to 4 parts of blood). PBMC were purified as described previously (17). Briefly, buffy coat cells were separated by centrifugation on Accu-Paque lymphocytes at a density of 1.086 g/ml (Accurate Chemical and Scientific Corp., Westbury, N.Y.). PBMC were washed thrice in Hanks' balanced salt solution (HBSS) (Sigma, St. Louis, Mo.) supplemented with 2% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, Utah) at 4°C. The cells were cultured in 96-well culture plates (Corning, Corning, N.Y.) seeded with 0.5 × 106 cells/well at a final density of 2.5 × 106 cells/ml in RPMI 1640 (Invitrogen, Carlsbad, Calif.) supplemented with 20% FBS-2 mM l-glutamine-100 U of penicillin/ml-100 μg of streptomycin/ml. DNA synthesis was assayed by incorporation of [3H]thymidine (Perkin-Elmer Life Sciences, Boston, Mass.) added in the amount of 1.0 mCi/well 48 h after cell culture commencement and 16 to 18 h prior to cell harvest. Cells were harvested on a semiautomated 96-well plate harvester (Skatron Inc., Sterling, Va.), and the amount of incorporated [3H]thymidine was determined by liquid scintillation spectroscopy (Packard Instrument Co., Downers Grove, Ill.) and expressed in counts per minute. The proliferation assays were done in quadruplicate, and the percentage of inhibition of proliferation was expressed as follows: (counts per minute of cultures with toxin/counts per minute of cultures without toxin) × 100.
Antibodies.
Polyclonal antibody to StxA1 was generated by a standard technique in New Zealand White rabbits. Murine monoclonal antibodies were obtained from the Washington State University Monoclonal Antibody Center (Pullman, Wash.). Antibodies specific for bovine B-cell markers recognized B-B1 (BAS9A; immunoglobulin M [IgM]), B-B2 (BAQ44A; IgM), or CD21-like (GB25A; IgG1) antigens, and antibody specific for T-cell markers recognized bovine CD3 (MM1A; IgG1). Monoclonal antibodies against BLV were specific for a capsid protein, p24 (MW3; IgG1), or for an envelope glycoprotein, gp51 (MW1; IgG1). The control antibody was mouse IgG1 specific for E. coli glycoproteins (coliS 69A).
BLV expression assay.
Two-milliliter aliquots of PBMC (0.5 × 106 cells/ml) were placed in culture dishes with or without 0.5 μg of toxin/ml. The harvested cultures were separated by centrifugation into cells and cell-free supernatants for analysis of cell-associated virus and cell-free virus, respectively. The cells were processed by repeated freeze-thaw cycles in 0.5 ml of 0.1 M Tris buffer (pH 7.5) with 0.1 M EDTA and 0.1 M phenylmethylsulfonyl fluoride, and complete lysis was determined microscopically. The cell lysates and the cell culture supernatants were transferred to nitrocellulose by using a 96-well blotter (Schleicher & Schuell, Keene, N.H.). The membranes were probed with antibodies against BLV proteins p24 and gp51, followed by anti-mouse antibody conjugated to alkaline phosphatase. Immunoblots were developed using 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium as the substrate, according to the manufacturer's instructions, and scanned with a Hewlett-Packard densitometer. The results were quantitated with the Molecular Analyzer analytical program. Cultures of PBMC from BLV-free cattle served as negative controls.
Toxin binding assays.
Purified StxA1 was iodinated using Iodo-Beads (Pierce Endogen, Rockford, Ill.). One bead was incubated with 0.5 mCi of carrier-free Nal25I (Amersham Pharmacia Biotech, Piscataway, N.J.) and 10 μg of StxA1 in 100 μl of 50 mM sodium phosphate (pH 7.4) for 7 min at room temperature. Labeled StxA1 was separated from free iodine by using a Bio-Gel P-10 column (Bio-Rad Laboratories, Hercules, Calif.) equilibrated with PBS. The specific radioactivity of the labeled toxin ranged from 3 to 30 μCi/μg. The iodinated toxin was tested for its enzymatic activity in a protein synthesis inhibition assay (see above) with unlabeled toxin as a control. PBMC, granulocytes, and erythrocytes from BLV-positive and BLV-negative cattle were incubated at concentrations of 2 × 107 cells/ml with 0.5 μg of the iodinated toxin/ml for 60 min at 4 or 37°C to prevent or to allow active internalization, respectively. The harvested cells were collected onto GF/B membranes (Whatman International Ltd., Maidstone, England) pre-soaked in 0.3% polyethyleneimine. The radioactivity of filter membranes (containing bound toxin) and of filtrates (containing free toxin) was measured in a COBRA II gamma counter (Packard Instrument Co.).
BLV virions were prepared from a BLV-infected bat lung cell line, BLV-bat2, as previously described (52). Virions were tested directly (dot blots), or the antigen was concentrated and fractioned electrophoretically on polyacrylamide gel and transferred to nitrocellulose. Blots were probed with StxA1 followed by rabbit polyclonal anti-StxA1 antibody and anti-rabbit antibody conjugated to alkaline phosphatase. Immunoblots were developed and scanned as described above for the BLV expression assay.
Flow cytometry.
Staining and formaldehyde fixation of cells for flow cytometric analysis was performed in 96-well plates by using a standard protocol (53). Second-step reagents were goat anti-mouse antibodies conjugated to fluorescein isothiocyanate, R-phycoerythrin, and Tricolor (Caltag, Burlingame, Calif.). Data were acquired on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) with a 488-nm argon laser. Viable and nonviable cells were gated on the basis of forward and side scatter. Results were analyzed using Becton Dickinson analytical software (CellQuest and Attractors).
Cell permeability assay.
Permeability of cells to macromolecules was assayed with fluorescein-conjugated lysine-fixable dextrans of 3-, 40-, and 70-kDa molecular mass (Molecular Probes, Eugene, Oreg.). One million cells suspended in 100 μl of RPMI 1640 with 10% FBS (RPMI-10% FBS) were added to a mixture containing 50 μl of 15 μg of primary antibody to cell surface molecules/ml of HBSS, 20 μl of 10 mg of dextran/ml of HBSS, and 30 μl of RPMI-10% FBS. Cells were incubated for 60 min on ice to allow passive uptake of dextrans, washed, incubated for 20 min on ice in 100 μl of RPMI-10% FBS with secondary antibodies, washed, and fixed with 2% formaldehyde in PBS. Control samples were incubated without dextrans or without second-step antibodies. All washes were done with PBS supplemented with 2% gamma-globulin-free horse serum. Cells exhibiting green fluorescence of 1.1 log or greater were considered positive for dextran. Typically, 50,000 cells were collected per sample.
Statistical analysis.
The results are presented as arithmetic means ± standard errors (SE) of three or more replicates. The results of the protein synthesis inhibition assays were analyzed using a linear regression. The results of the permeability assays were analyzed by analysis of variance, after cosign transformation of percentages expressed as values ranging from 0 to 1, using MINITAB statistical analysis software (Minitab, Inc.).
RESULTS
Stx2 had antiviral activity similar to that of Stx1.
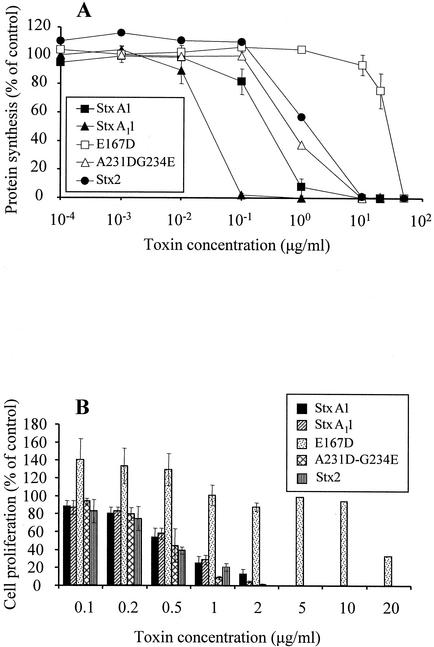
To determine whether antiviral activity is common to the Stxs prevalent in cattle, Stx2 holotoxin (∼70 kDa) was tested for its ability to suppress BLV-dependent cell proliferation and compared to that of StxA1 (∼32 kDa). To ensure that the toxins were enzymatically active, they were tested in a protein synthesis inhibition assay. On a molar basis, Stx2 was 2.5-fold less enzymatically active than StxA1 when compared for 50% protein synthesis inhibition (regression analysis; data not shown) (Fig. 1A). Antiviral activity was measured as inhibition of SLP (Fig. 1B). Stx2 had slightly more antiviral activity than StxA1, since fourfold less Stx2 than StxA1 (on a molar basis) inhibited cell proliferation by 50%. These results indicate that Stx1 and Stx2 had similar enzymatic and antiviral properties. Although the antiviral effects of Stx in vitro do not require the Gb3-binding B subunit (17), both Stx1 and Stx2 holotoxins were more potent on a molar basis than the A chain alone. This could be due to numerous factors that we did not measure, such as differential proteolysis, loss of enzymatic activity, and/or differences in cell internalization.
FIG. 1.
Comparison of Stx2 and StxA1 mutant enzymatic and antiviral activities. Enzymatic and antiviral activities of Stx2, three mutant toxins, and StxA1 were compared in protein synthesis (A) and cell proliferation (B) inhibition assays. Rabbit reticulocyte lysates were preincubated with various amounts of StxA1, Stx2 holotoxin, A-B association mutant (StxA11), enzymatic mutant (E167D), or cell-trafficking mutant (A231G-D234E) or with no toxin, and these lysates were then used in a luciferase protein synthesis assay. Toxin enzymatic activity was expressed as a percentage of that of the control and was calculated by dividing the amount of luciferase made by lysates incubated with toxin by the amount of luciferase made by lysates without toxin. PBMC from BLV-positive cattle were cultured for 72 h with various amounts of StxA1 or Stx2 holotoxin or with no toxin. Antiviral activity was expressed as a percentage of that of the control and was calculated by dividing the amounts of [3H]thymidine incorporated by PBMC cultured with toxins by the amounts incorporated by PBMC cultured without toxin. Data are means ± SE from two experiments performed in duplicate (protein synthesis inhibition) or four experiments performed in quadruplicate (PBMC proliferation).
Enzymatically active StxA1 mutants retained antiviral activity in spite of a loss of receptor-mediated toxicity.
The observation that the antiviral activity of Stx1 did not require the Stx1 B subunit (17) prompted us to investigate whether mutations of StxA1 that abolish receptor-mediated cytotoxicity towards Vero cells and other Gb3-expressing cells (54) would also affect the antiviral activity of StxA1. Thus, we analyzed the ability of three well-defined StxA1 mutants to inhibit protein synthesis and suppress SLP (Fig. 1). The enzymatic mutant E167D had 300-fold less activity than StxA1 when compared for 50% inhibition of protein synthesis, and its ability to inhibit protein synthesis was significantly different from that of the other toxins at concentrations ranging from 1.0 to 20 μg/ml (P < 0.01, analysis of variance) (Fig. 1A). The enzymatic activities of the A-B association mutant StxA11 and the cell-trafficking mutant A231D-G234E were both similar to that of wild-type StxA1 (Fig. 1A). StxA11 required a 2.3-fold-lower molar concentration than wild-type StxA1, and A231D-G234E required the same molar concentration as wild-type StxA1 for 50% inhibition of protein synthesis.
The antiviral activity of the toxins was tested in a SLP suppression assay and expressed as the ability to suppress BLV-dependent cell proliferation (Fig. 1B). The antiviral activity of the mutant toxins was associated with enzymatic activity but not with other functional characteristics. The mutants that retained undiminished enzymatic activity (StxA11 and A232D-G234E) suppressed SLP as effectively as wild-type StxA1. Thus, neither of the mutations located outside of the catalytic center decreased antiviral activity of the toxins, in spite of abolishing the ability of mutant toxin to associate with B subunits (StxA11) or to undergo retrograde transport within intoxicated cells (A232D-G234E). The less-active enzymatic mutant E167D had reduced antiviral activity and required a 40-fold-higher concentration than other toxins to inhibit cell proliferation by 50%. Interestingly, this molecule not only did not inhibit cell proliferation at concentrations ≤10 μg/ml but (at concentrations ranging from 0.1 to 0.5 μg/ml) consistently increased proliferation by 35 to 40% compared to the control.
StxA1 enzymatic activity was required for suppression of BLV protein expression.
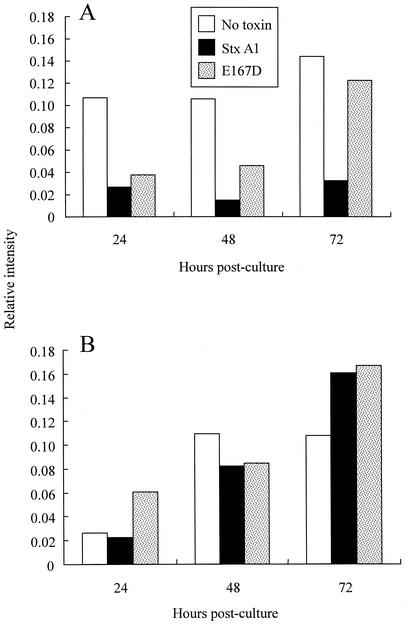
The impact of StxA1 on viral protein expression was assessed by measuring the amounts of BLV proteins p24 and gp51 in cell culture. PBMC from BLV-positive cattle were cultured with wild-type StxA1 or with enzymatic mutant E167D or without toxin, and BLV proteins were measured. Harvested cultures were separated into cells and cell-free supernatants to measure cell-associated and cell-free viral proteins, respectively (Fig. 2). Cell-associated BLV proteins were not detectable in ex vivo PBMC by immunoblotting (data not shown) but could be detected 12 h postculture (relative intensity of 0.04; data not shown). At 24 h postculture and later, cell-associated BLV proteins were prominent in control cultures not treated with toxin and apparent in cultures treated with the enzymatic mutant E167D but barely detectable in cultures treated with StxA1 (Fig. 2A).
FIG. 2.
Effect of the presence of StxA1 and the enzymatic mutant E167D on BLV expression by cultured PBMC from BLV-positive cattle. PBMC from BLV-positive cattle were cultured with StxA1 (1.0 μg/ml) or enzymatic mutant E167D (1.0 μg/ml) or without toxin and were harvested at 24, 48, and 72 h postculture. Cells (A) and culture supernatants (B) were assayed for expression of BLV proteins p24 and gp51 by blotting the lysed cells or cell-free culture supernatants onto nitrocellulose and probing with mouse monoclonal antibodies to p24 and gp51 followed by anti-mouse Ig antibody conjugated to alkaline phosphatase. Blots were developed using 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium as the substrate, and the reaction intensities were quantified using a densitometer. PBMC from BLV-negative cattle were used as controls. The experiment was performed four times with PBMC from three BLV-positive cattle, and the results of a representative experiment are shown.
In contrast to BLV protein expression associated with cells, the cell-free supernatants from cultures incubated with toxins contained amounts of BLV proteins either similar to (48 h) or greater than (72 h) those found in the control cultures. The finding that StxA1-treated cultures harvested at 48 h and 72 h contained small amounts of BLV proteins associated with cells but high amounts of BLV proteins in the culture supernatants suggests that the interaction of StxA1 with target cells interrupted virion assembly and induced cell death and/or loss of membrane integrity to a much greater extent than that of E167D. Such a possibility would be consistent with our results of flow cytometric analysis, which suggested that the percentage of BLV-expressing cells was reduced in cultures treated with StxA1 but not in cultures treated with the enzymatic mutant E167D or without toxin. However, due to a scarcity of BLV protein-positive cells among the PBMC from BLV-positive cattle (<2% in uncultured ex vivo PBMC), the results regarding the changes in prevalence of BLV-expressing cells were inconclusive and are not shown.
StxA1 did not bind to bovine blood cells or to viral particles.
To test whether StxA1 binds to corpuscular blood components, whole blood from BLV-positive and BLV-negative cattle was fractionated by density centrifugation into PBMC, granulocytes, and erythrocytes and the fractions were incubated with 125I-labeled StxA1. The enzymatic activity of radiolabeled toxin was similar to that of the unlabeled toxin in the protein synthesis inhibition assay, indicating that iodination did not disrupt the molecular structure of StxA1 (data not shown). Leucocytes and erythrocytes from BLV-negative and BLV-positive cattle did not bind appreciable amounts of the radiolabeled toxin (data not shown). Notwithstanding that StxA1 entry into target cells would be necessary for suppression of SLP or viral protein synthesis, it is likely that direct assessment of toxin entry into cells using radiolabeled toxin was beyond the limits of sensitivity of the assay due to the scarcity of BLV-expressing cells (<2% in ex vivo PBMC). In addition, toxin binding to virions was assessed in dot blots of BLV virus and immunoblots of concentrated viral antigens probed with StxA1. No binding was detected (data not shown). Thus, the results of all toxin binding measurements support the contention that StxA1 interacts with selected and rare cellular targets in PBMC cultures.
BLV-expressing cells were highly permeable to macromolecules.
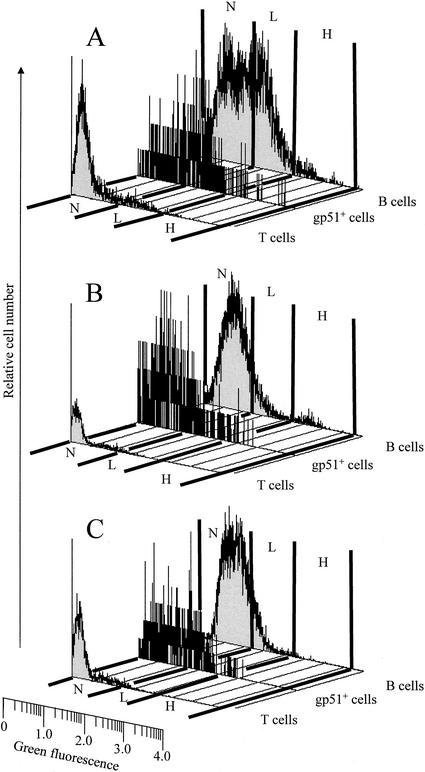
Although we have not excluded the possibility of receptor-mediated binding of the A subunit to bovine PBMC, the finding that suppression of BLV-dependent SLP occurred in cultures treated with either the isolated Stx1 A chain (devoid of receptor-binding B subunits) or with the cell-trafficking mutant A231D-G234E (crippled in retrograde transport) strongly indicated that the toxin enters the cytosol of target cells directly. Because virally infected cells often exhibit increased permeability to macromolecules due to virus-induced alterations in the cell membrane (reviewed in reference 9), we hypothesized that entry of StxA1 into the relatively rare cellular targets in PBMC cultures involved increased permeability of the cells expressing BLV. Accordingly, the permeability of PBMC from BLV-positive cattle was assessed using flow cytometry. The cells were stained with Tricolor-labeled monoclonal antibody MW1, specific for BLV surface unit glycoprotein (51,000 molecular weight [gp51]), and incubated with fluorescein-conjugated 40-kDa dextran to compare the permeability of PBMC negative for gp51 to that of PBMC positive for gp51. Cells expressing gp51 on their surfaces are engaged in virion assembly; cells not expressing gp51 are not expressing virus. In three separate experiments, PBMC from three BLV-positive cattle were gated on the basis of gp51 expression and their levels of permeability were assessed by measuring green fluorescence. Cells exhibiting green fluorescence above 1.1 log were considered dextran positive and were divided arbitrarily into groups of cells exhibiting low fluorescence (1.1 to 2.05 log) and high fluorescence (2.05 to 3.7 log). Cell not expressing virus (gp51 negative) were rarely permeable to dextran, as 2.2, 10.1, and 13.7% of these cells exhibited low levels of green fluorescence and 0.8, 2.2, and 1.1% of these cells exhibited high levels of green fluorescence (values from each of the three animals, respectively). In contrast, cells expressing virus (gp51 positive) consistently internalized dextran, as 9.8, 20.0, and 33.8% of these cells exhibited low levels of green fluorescence and 14.1, 16.6, and 15.2% exhibited high levels of green fluorescence (values from each of the three animals, respectively). These data are representative of three experiments per animal.
Because of the high degree of BLV tropism for B cells, gp51-positive PBMC are comprised primarily of B lymphocytes whereas gp51-negative PBMC are comprised of B lymphocytes and other categories of mononuclear blood cells. Thus, comparing permeability levels of total PBMC could produce biased results, since BLV rarely infects cells other than B lymphocytes (16, 48). Accordingly, we compared the permeability of the total B-cell population with the permeability of gp51-positive cells and T cells by using dextrans of 3-, 40-, and 70-kDa molecular mass (Fig. 3). The fact that similar proportions of gp51-positive cells were permeable to 40- and 70-kDa dextrans (Fig. 3B and C and data not shown) indicates that direct absorption of toxin into the cytosol via the cell membrane is an entry route potentially accessible to the isolated A chain of Stx1 (∼32 kDa) and also to Stx1 holotoxin (∼70 kDa).
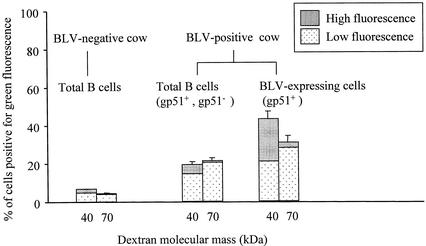
FIG. 3.
Permeability of T cells, B cells, and gp51-positive PBMC from BLV-positive cattle. PBMC from BLV-positive cattle were incubated for 1 h with 3-kDa (A), 40-kDa (B), or 70-kDa (C) fluorescein-conjugated dextrans (green fluorescence) and analyzed by flow cytometry. Lymphocyte subpopulations were identified on the basis of staining with Tricolor-labeled monoclonal antibodies (red fluorescence) as T cells (positive for bovine CD3 and comprising 26% of the total cells), B cells (positive for CD21-like antigen and comprising 48% of the total cells), and gp51-positive cells (BLV-expressing cells positive for viral gp51 antigen and comprising 1.6% of the cells) (designations at right of each graph). Cells were assessed for dextran content by measuring green fluorescence; cells exhibiting fluorescence below 1.1 log were considered dextran negative (N) and cells exhibiting fluorescence above 1.1 log were considered dextran positive and were divided into groups of cells exhibiting low fluorescence (L; 1.1 to 2.05 log) or high fluorescence (H; 2.05 to 3.7 log). Data are histograms of dot plots (50,000 cells per sample) from a representative experiment with one BLV-positive cow. The cell numbers are relative and are not comparable between histograms, because the individual histograms were scaled independently.
The permeability of total B cells from BLV-positive cattle to dextrans was lower than the permeability of gp51-positive cells but higher than the permeability of T cells, which rarely exhibited green fluorescence above background, even after incubation with low-molecular-mass dextran (Fig. 3A). Since the B cells from BLV-positive cattle with persistent lymphocytosis exhibit abnormal characteristics (50, 51) and cannot be considered normal, it was important to compare the permeability of B cells from BLV-positive cattle with the permeability of B cells from BLV-negative cattle (Fig. 4). The latter cells consistently showed miniscule permeability to dextrans whereas the permeability of total B cells from BLV-positive cattle was consistently greater, indicating that not only BLV-expressing cells but also some other B cells from these animals may have been permeable and sensitive to toxin.
FIG. 4.
Permeability of B lymphocytes from BLV-negative and BLV-positive cattle. PBMC from BLV-negative or BLV-positive cattle were incubated for 1 h with 40- or 70-kDa fluorescein-conjugated dextrans (green fluorescence) and analyzed by flow cytometry. On the basis of staining with Tricolor labeled monoclonal antibodies (red fluorescence), lymphocyte subpopulations were identified in separate samples as B cells (positive for CD21-like antigen) and as BLV-expressing cells (positive for viral gp51 antigen) and assessed for dextran content by measuring green fluorescence. Cells exhibiting fluorescence above 1.1 log were considered dextran positive and were divided into groups of cells exhibiting low fluorescence (1.1 to 2.05 log) or high fluorescence (2.05 log to 3.7 log). Results are percentages of cells in each category exhibiting green fluorescence + SE. Data are from a representative experiment performed in triplicate.
DISCUSSION
This study supported the hypothesis that STEC colonization of the gastrointestinal tract may benefit ruminant hosts by the ability of Stxs to exert antiviral activity. We showed that (i) antiviral activity was common to Stx1 and Stx2, (ii) the catalytic center was required for antiviral activity, (iii) StxA1 mutants unable to kill cells via the classical receptor-mediated route had undiminished antiviral activity, (iv) StxA1 did not bind to bovine blood cells or to free virus, (v) Stx activity was targeted to BLV-expressing B lymphocytes, and (vi) BLV-expressing B lymphocytes were highly permeable to macromolecules.
We used natural BLV infections in cattle as a model to study the impact of toxin on viral activity. Removal of PBMC from autologous serum containing specific antibody against BLV precipitates a chain of events in which provirus becomes derepressed and viral protein synthesis and virus release occur. The number of cells expressing virus ex vivo is <2%, and upon culturing this proportion may increase. Viral derepression is accompanied by SLP, a rapid proliferation of a small number of B cells (and some T cells) that are not expressing virus. The highest number of replicating cells can be measured 72 h postculture. SLP can be blocked by treating cultures with toxin within 12 h, but the toxin has little effect if added later (17). Thus, toxin interferes with the initiation of SLP but has little effect on the subsequent proliferation of cells that do not express virus. Our hypothesis is that only the rare BLV-expressing cells are sensitive to toxin because viral synthesis increases membrane permeability of these cells, allowing toxin entry with ensuing inhibition of viral protein synthesis and elimination of SLP. Our findings in vitro suggest that Stx is a factor in limiting BLV infection in cattle to the status of a chronic well-tolerated disease rather than an acute deadly disease.
The finding that both Stx1 and Stx2 had antiviral activity in vitro buttresses a conjecture of antiviral activity in animals carrying STEC. This finding is consistent with research showing the cytotoxic activity of plant RIPs against virally infected animal cells (18, 19) and work showing that RIPs have antiviral activity for the plants that synthesize them (49). Cattle are transiently colonized at various times by STEC expressing Stx1, Stx2, and/or Stx2 variants in some combination. Toxins can be detected in fecal samples from cattle, indicating that STEC express toxin in vivo (30), and individual cattle are likely exposed to Stxs in their gastrointestinal tracts. Stxs translocate through the human intestinal epithelium (1) and therefore may have a similar ability in the bovine gastrointestinal mucosa. The notable lack of detrimental effects of the presence of Stx in cattle is likely due to the absence of Gb3 in the bovine vasculature (46); however, the recent finding of Gb3 on bovine crypt epithelial cells in the small and large intestine indicate that Stxs binding plays a role in STEC intestinal colonization (27) and may facilitate transport of toxin systemically. Reports of cattle colostral antibodies to Stx1 and Stx2 (44) indicate that the toxins leave the bovine intestinal lumen and are processed by the cells of the mucosa-associated lymphatic system. Also, reports of the presence of neutralizing antibodies to Stx1 in serum indicate systemic exposure to this toxin (32, 44); possibly, these antibodies remove toxin in sensitized animals. However, anti-Stx2 neutralizing antibodies are not detected in serum (32, 44); therefore, this toxin does not enter the circulation or, more likely, does not elicit a systemic immune response. Thus, lymphocytes may interact with Stx in the intestinal mucosa and/or in the systemic circulation.
The finding that the enzymatic activity of Stx was necessary for antiviral activity is similar to that of data showing that plant RIPs must maintain enzymatic activity to exert antiviral impact (49, 57, 58). We did not identify the substrate for this enzymatic activity, but it may be the host cell ribosome or viral nucleic acid(s). Recent studies showed that mutant pokeweed antiviral proteins devoid of antiribosomal activity but capable of depurination of capped mRNA transcripts retain antiviral activity (29). We demonstrated the requirement for enzymatic activity by using the well-characterized E167D mutant that contains a conservative substitution (aspartic acid for a glutamic acid at position 167) to disable the catalytic center without significantly altering molecular integrity (28).
The A-B association mutant StxA11 has enhanced enzymatic activity because it lacks 38 carboxy-terminal amino acids (2) that block the catalytic center in the full-length A chain (22). In spite of the fact that StxA11 was enzymatically more active than wild-type StxA1 or the cell-trafficking mutant A231D-G234E, it did not have more antiviral activity than these toxins. Although we did not measure toxin stability, the A-B association mutant StxA11 may have been more susceptible to degradation in culture medium and/or inside intoxicated cells than the other toxins. Also, our assay method may not have been sensitive enough to detect differences in antiviral activity that might result from severalfold differences in enzymatic activity.
The molecular motifs required for receptor-mediated cytotoxicity of Stxs were not necessary for antiviral effect. Both the StxA11 mutant (unable to associate with B subunits) and the A231D-G234E mutant (crippled in cell-trafficking ability) had antiviral activity similar to that of a wild-type StxA1. These results are consistent with the antiviral activity of the ricin A chain and of RIP hemitoxins (39, 43). Furthermore, antiviral activity of StxA11 and of A231D-G234E mutants, which are devoid of receptor-mediated cytotoxicity towards Vero cells, pointed to the direct absorption of toxins by virus-expressing cells as a possible mechanism of target cell intoxication.
We showed that the numbers of BLV-expressing cells (i.e., cells positive for the presence of BLV protein gp51 on their surfaces) were reduced in cultures treated with Stx. Although the identity of the cells targeted by Stxs in the course of SLP suppression was not unequivocally established, previous results indicate that the antiviral action of Stxs is not indiscriminate and targets select and infrequently occurring cells (17). This determination is supported by the present findings that StxA1 did not bind to bovine erythrocytes, bovine leukocytes, or free viruses. Flow cytometric analysis suggested that the BLV-expressing cells in PBMC cultures are eliminated by StxA1, but we were unable to detect absorption of radiolabeled StxA1 by the BLV-expressing cells or any other cells in PBMC cultures. We ascribe this failing to the following constraints. First, the proportion of BLV-expressing cells (the presumptive targets) in culture was very low, limiting our ability to analyze them directly. Second, the toxin exerts a lethal cytotoxic effect at extremely low intracellular concentrations and may have killed the target cells before they accumulated a detectable amount of the toxin. Third, the loss of BLV-expressing cells during incubations and washings was proportionally greater than the loss of other cells.
Since viruses are known to increase cell membrane permeability of infected cells (9), we hypothesized that increased permeability of BLV-expressing cells to toxins may be involved in SLP suppression and may explain the sensitivity of these cells to Stx. Although we did not measure toxin entry into cells, we did show that BLV-expressing cells were permeable to 70-kDa molecules and that the permeability of B lymphocytes from cattle with BLV-induced persistent lymphocytosis greatly exceeded the permeability of B cells from BLV-negative cattle. From these results we surmise that the cells that express BLV constitute the primary targets of Stxs. In addition, some B cells from BLV-positive cattle (not expressing BLV but permeable to macromolecules) may be secondary target cells, since most of these cells contain BLV provirus (40) and are physiologically abnormal (50, 51).
Acknowledgments
This work was supported, in part, by the Idaho Agriculture Experiment Station, U.S. Department of Agriculture NRICGP grant 99-35201-8539, and Public Health Service grants NO1-HD-0-3309 and P20RR15587 from the National Institutes of Health.
We thank Linda L. Norton for help with providing blood from BLV-infected cows. We thank Alison D. O'Brien and Edda Twiddy for providing purified Stx2 holotoxin.
I.B. and W.A.F. contributed equally to this publication.
Editor: A. D. O'Brien
REFERENCES
- 1.Acheson, D. W., R. Moore, S. De Breucker, L. Lincicome, M. Jacewicz, E. Skutelsky, and G. T. Keusch. 1996. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 64:3294-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin, P. R., P. E. Jablonski, G. A. Bohach, A. K. Dunker, and C. J. Hovde. 1994. Evidence that the A2 fragment of Shiga-like toxin type I is required for holotoxin integrity. Infect. Immun. 62:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliga, V., and J. F. Ferrer. 1977. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc. Soc. Exp. Biol. Med. 156:388-391. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri, L., M. G. Battelli, and F. Stirpe. 1993. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1154:237-282. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, B. B., N. J. Burns III, K. J. Park, M. I. Dawson, M. Kende, and R. W. Sidwell. 1991. Antiviral immunotoxins: antibody-mediated delivery of gelonin inhibits Pichinde virus replication in vitro. Antivir. Res. 15:125-138. [DOI] [PubMed] [Google Scholar]
- 6.Bendixen, H. J. 1965. Bovine enzootic leukosis. Adv. Vet. Sci. 10:129-204. [PubMed] [Google Scholar]
- 7.Bertin, Y., K. Boukhors, N. Pradel, V. Livrelli, and C. Martin. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J. Clin. Microbiol. 39:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutin, L., D. Geier, H. Steinruck, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco, L. 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res. 45:61-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerqueira, A. M., B. E. Guth, R. M. Joaquim, and J. R. Andrade. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121. [DOI] [PubMed] [Google Scholar]
- 11.Chang, M. C., S. K. Saksena, I. F. Lau, and Y. H. Wang. 1979. Induction of mid-term abortion by trichosanthin in laboratory animals. Contraception 19:175-184. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold, R., and P. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 13.Donohue-Rolfe, A., D. W. Acheson, and G. T. Keusch. 1991. Shiga toxin: purification, structure, and function. Rev. Infect. Dis. 13(Suppl. 4):S293-S297. [DOI] [PubMed] [Google Scholar]
- 14.Dosio, F., P. Brusa, L. Delprino, G. Grosa, M. Ceruti, and L. Cattel. 1994. A new approach in the synthesis of immunotoxins: ribosome inactivating protein noncovalently bound to monoclonal antibody. J. Pharm. Sci. 83:206-211. [DOI] [PubMed] [Google Scholar]
- 15.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteban, E. N., R. M. Thorn, and J. F. Ferrer. 1985. Characterization of the blood lymphocyte population in cattle infected with the bovine leukemia virus. Cancer Res. 45:3225-3230. [PubMed] [Google Scholar]
- 17.Ferens, W. A., and C. J. Hovde. 2000. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect. Immun. 68:4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Puentes, C. 1984. Permeability to inhibitors of protein synthesis in virus infected cells. Mol. Biol. Rep. 10:65-68. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Puentes, C., and L. Carrasco. 1980. Viral infection permeabilizes mammalian cells to protein toxins. Cell 20:769-775. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer, J. F. 1980. Bovine lymphosarcoma. Adv. Vet. Sci. Comp. Med. 24:1-68. [PubMed] [Google Scholar]
- 21.Ferrer, J. F., R. R. Marshak, D. A. Abt, and S. J. Kenyon. 1979. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J. Am. Vet. Med. Assoc. 175:705-708. [PubMed] [Google Scholar]
- 22.Fraser, M. E., M. M. Chernaia, Y. V. Kozlov, and M. N. James. 1994. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat. Struct. Biol. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 23.Gansheroff, L. J., and A. D. O'Brien. 2000. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA 97:2959-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girbes, T., J. M. Ferreras, R. Iglesias, L. Citores, C. De Torre, M. L. Carbajales, P. Jimenez, F. M. De Benito, and R. Munoz. 1996. Recent advances in the uses and applications of ribosome-inactivating proteins from plants. Cell Mol. Biol. (Noisy-le-grand) 42:461-471. [PubMed] [Google Scholar]
- 25.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 26.Hartley, M. R., G. Legname, R. Osborn, Z. Chen, and J. M. Lord. 1991. Single-chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 290:65-68. [DOI] [PubMed] [Google Scholar]
- 27.Hoey, D. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51:143-149. [DOI] [PubMed] [Google Scholar]
- 28.Hovde, C. J., S. B. Calderwood, J. J. Mekalanos, and R. J. Collier. 1988. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc. Natl. Acad. Sci. USA 85:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudak, K. A., P. Wang, and N. E. Tumer. 2000. A novel mechanism for inhibition of translation by pokeweed antiviral protein: depurination of the capped RNA template. RNA 6:369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyatt, D. R., J. C. Galland, and J. R. Gillespie. 2001. Usefulness of a commercially available enzyme immunoassay for Shiga-like toxins I and II as a presumptive test for the detection of Escherichia coli O157:H7 in cattle feces. J. Vet. Diagn. Investig. 13:71-73. [DOI] [PubMed] [Google Scholar]
- 31.Jacewicz, M., H. Clausen, E. Nudelman, A. Donohue-Rolfe, and G. T. Keusch. 1986. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 163:1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, R. P., W. C. Cray, Jr., and S. T. Johnson. 1996. Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect. Immun. 64:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junqua, S., P. Wils, Z. Mishal, and J. B. Le Pecq. 1987. Comparison of inhibitory effect of galactose analogs on the binding and cytotoxicity of an anti-globotriaosylceramide monoclonal antibody coupled or not coupled to pokeweed antiviral protein. Eur. J. Immunol. 17:459-464. [DOI] [PubMed] [Google Scholar]
- 34.Kaddu-Mulindw, D. H., T. Aisu, K. Gleier, S. Zimmermann, and L. Beutin. 2001. Occurrence of Shiga toxin-producing Escherichia coli in fecal samples from children with diarrhea and from healthy zebu cattle in Uganda. Int. J. Food Microbiol. 66:95-101. [DOI] [PubMed] [Google Scholar]
- 35.Khan, A., S. Yamasaki, T. Sato, T. Ramamurthy, A. Pal, S. Datta, N. R. Chowdhury, S. C. Das, A. Sikdar, T. Tsukamoto, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 2002. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg. Infect. Dis. 8:54-62. [PubMed] [Google Scholar]
- 36.Kobayashi, H., J. Shimada, M. Nakazawa, T. Morozumi, T. Pohjanvirta, S. Pelkonen, and K. Yamamoto. 2001. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl. Environ. Microbiol. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokai-Kun, J. F., A. R. Melton-Celsa, and A. D. O'Brien. 2000. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J. Biol. Chem. 275:3713-3721. [DOI] [PubMed] [Google Scholar]
- 38.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1996. Escherichia coli O157:H7 in microbial flora of sheep. J. Clin. Microbiol. 34:431-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee-Huang, S., P. L. Huang, H. F. Kung, B. Q. Li, P. Huang, H. I. Huang, and H. C. Chen. 1991. TAP 29: an anti-human immunodeficiency virus protein from Trichosanthes kirilowii that is nontoxic to intact cells. Proc. Natl. Acad. Sci. USA 88:6570-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirsky, M. L., C. A. Olmstead, Y. Da, and H. A. Lewin. 1996. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J. Virol. 70:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montenegro, M. A., M. Bülte, T. Trumpf, S. Aleksiæ, G. Reuter, E. Bulling, and R. Helmuth. 1990. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J. Clin. Microbiol. 28:1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 43.Olson, M. C., S. Ramakrishnan, and R. Anand. 1991. Ribosomal inhibitory proteins from plants inhibit HIV-1 replication in acutely infected peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 7:1025-1030. [DOI] [PubMed] [Google Scholar]
- 44.Pirro, F., L. H. Wieler, K. Failing, R. Bauerfeind, and G. Baljer. 1995. Neutralizing antibodies against Shiga-like toxins from Escherichia coli in colostra and sera of cattle. Vet. Microbiol. 43:131-141. [DOI] [PubMed] [Google Scholar]
- 45.Pradel, N., V. Livrelli, C. De Champs, J.-B. Palcoux, A. Reynaud, F. Scheutz, J. Sirot, B. Joly, and C. Forestier. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 97:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandvig, K., M. Ryd, O. Garred, E. Schweda, P. K. Holm, and B. van Deurs. 1994. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 126:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz, I., A. Bensaid, B. Polack, B. Perrin, M. Berthelemy, and D. Levy. 1994. In vivo leukocyte tropism of bovine leukemia virus in sheep and cattle. J. Virol. 68:4589-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stirpe, F., L. Barbieri, M. G. Battelli, M. Soria, and D. A. Lappi. 1992. Ribosome-inactivating proteins from plants: present status and future prospects. Bio/Technology 10:405-412. [DOI] [PubMed] [Google Scholar]
- 50.Stone, D. M., A. J. Hof, and W. C. Davis. 1995. Up-regulation of IL-2 receptor alpha and MHC class II expression on lymphocyte subpopulations from bovine leukemia virus infected lymphocytotic cows. Vet. Immunol. Immunopathol. 48:65-76. [DOI] [PubMed] [Google Scholar]
- 51.Stone, D. M., T. F. McElwain, and W. C. Davis. 1994. Enhanced B-lymphocyte expression of IL-2R alpha associated with T lymphocytosis in BLV-infected persistently lymphocytotic cows. Leukemia 8:1057-1061. [PubMed] [Google Scholar]
- 52.Stone, D. M., L. K. Norton, J. C. Chambers, and W. J. Meek. 2000. CD4 T lymphocyte activation in BLV-induced persistent B lymphocytosis in cattle. Clin. Immunol. 96:280-288. [DOI] [PubMed] [Google Scholar]
- 53.Stone, D. M., L. K. Norton, and W. C. Davis. 2000. Spontaneously proliferating lymphocytes from bovine leukaemia virus-infected, lymphocytotic cattle are not the virus-expressing lymphocytes, as these cells are delayed in G(0)/G(1) of the cell cycle and are spared from apoptosis. J. Gen. Virol. 81:971-981. [DOI] [PubMed] [Google Scholar]
- 54.Suhan, M. L., and C. J. Hovde. 1998. Disruption of an internal membrane-spanning region in Shiga toxin 1 reduces cytotoxicity. Infect. Immun. 66:5252-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takashima, I., and C. Olson. 1981. Relation of bovine leukosis virus production on cell growth cycle. Arch. Virol. 69:141-148. [DOI] [PubMed] [Google Scholar]
- 56.Thorn, R. M., P. Gupta, S. J. Kenyon, and J. F. Ferrer. 1981. Evidence that the spontaneous blastogenesis of lymphocytes from bovine leukemia virus-infected cattle is viral antigen specific. Infect. Immun. 34:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tumer, N. E., D. J. Hwang, and M. Bonness. 1997. C-terminal deletion mutant of pokeweed antiviral protein inhibits viral infection but does not depurinate host ribosomes. Proc. Natl. Acad. Sci. USA 94:3866-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, P., and N. E. Tumer. 2000. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 55:325-355. [DOI] [PubMed] [Google Scholar]
- 59.Yeung, H. W., W. W. Li, Z. Feng, L. Barbieri, and F. Stirpe. 1988. Trichosanthin, alpha-momorcharin and beta-momorcharin: identity of abortifacient and ribosome-inactivating proteins. Int. J. Pept. Protein Res. 31:265-268. [DOI] [PubMed] [Google Scholar]
- 60.Zollman, T. M., P. R. Austin, P. E. Jablonski, and C. J. Hovde. 1994. Purification of recombinant Shiga-like toxin type I A1 fragment from Escherichia coli. Protein Expr. Purif. 5:291-295. [DOI] [PubMed] [Google Scholar]