Abstract
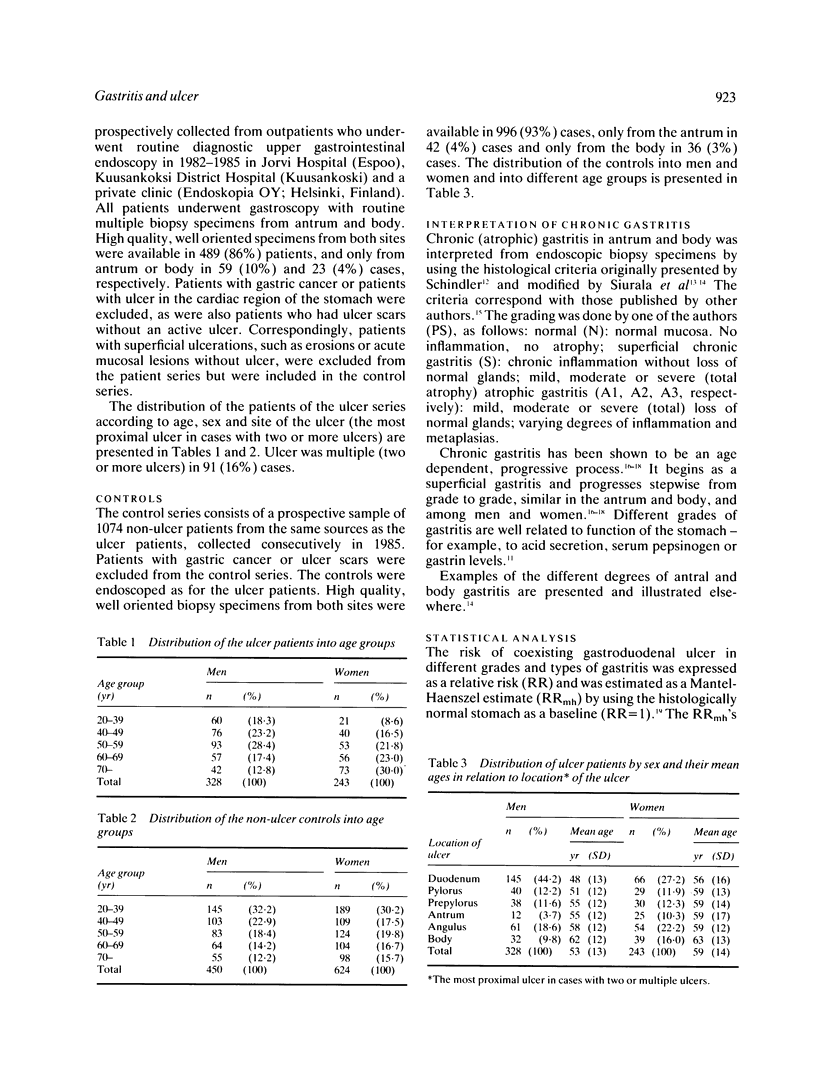
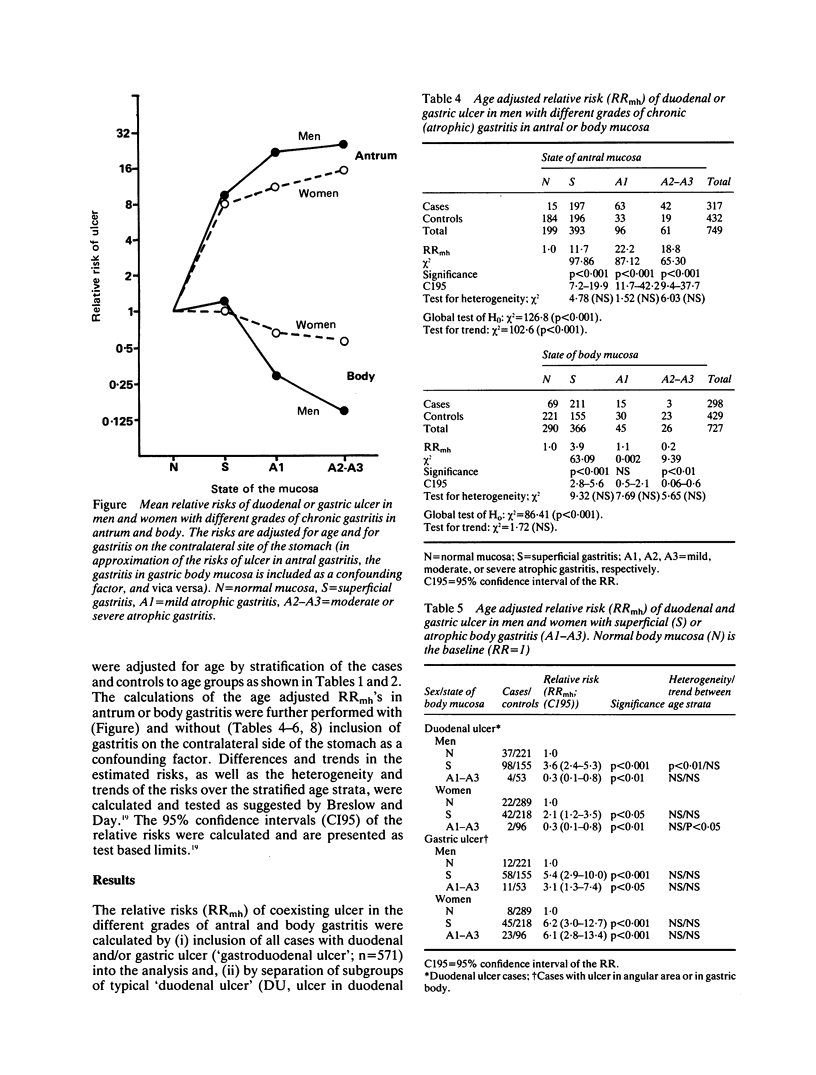
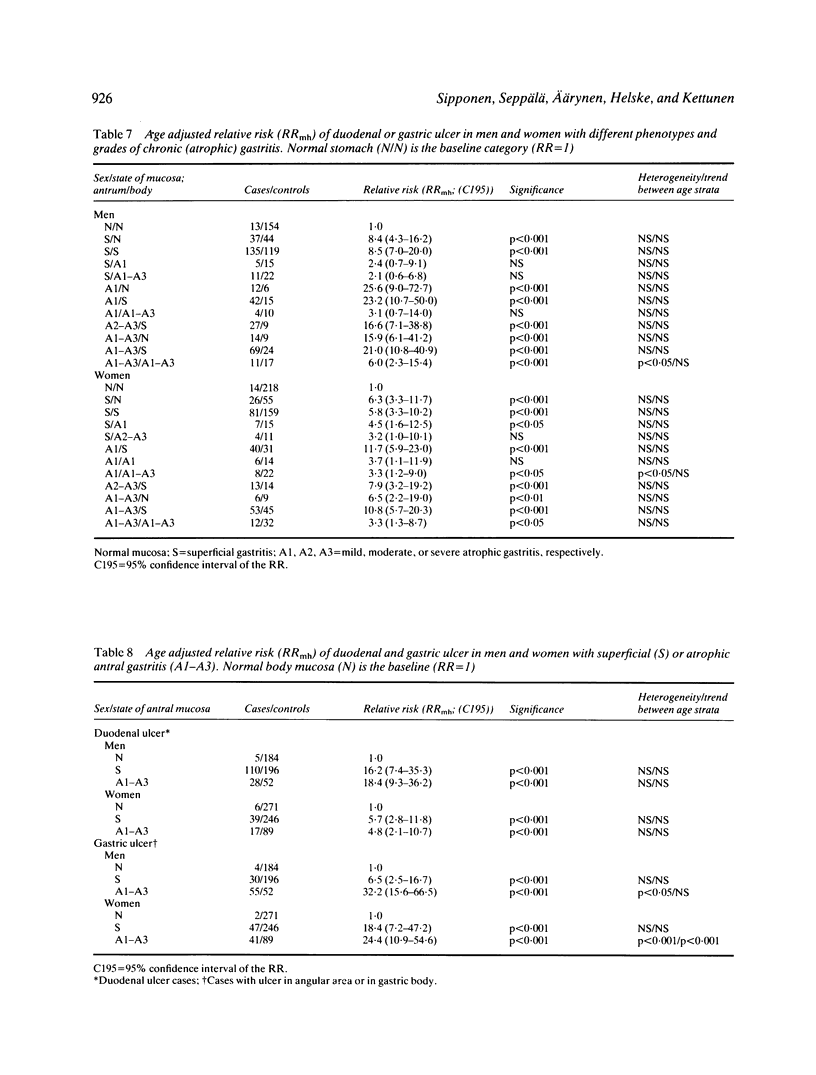
Chronic (atrophic) gastritis (AG) is common in active duodenal (DU) and gastric ulcer (GU) disease. In this case control study in consecutive prospective outpatients (571 cases and 1074 controls) who had undergone diagnostic upper gastrointestinal endoscopy and routine biopsies from both antral and body mucosa, we calculated the risk of coexisting active DU and/or GU in different gastritis of the antrum or body and according to grade (superficial gastritis, mild, moderate or severe atrophic gastritis). The risk of coexisting active gastroduodenal ulcer (ulcer in duodenum and/or stomach), as well as the risk of DU or GU, was dependent upon the presence and grade of gastritis in antrum and body mucosa. The risk of coexisting ulcer, as expressed as an age adjusted relative risk (RR) and calculated as odds ratio of gastritis in cases and controls, was significantly increased in the presence of superficial antral and body gastritis (RR = 8.5 (7.0-20.0) in men; RR = 5.8 (3.3-10.2) in women), as compared with the risk of ulcer in subjects with histologically normal mucosa (RR = 1). The risk of ulcer, and the risk of GU in particular, increased further with increasing severity of antral gastritis. In such patients with moderate or severe atrophic antral gastritis the RR of coexisting ulcer even exceeded 20 in men and 10 in women (RR = 25.6 (9.0-72.7) in men; RR = 11.7 (5.9-23.0) in women). On the other hand, the RR of ulcer, and the RR of DU in particular, was below 1 in the presence of atrophic gastritis in the gastric body, irrespective of the grade of gastritis in the antrum. We conclude that the type and grade of gastritis strongly predicts the risk of coexisting peptic ulcer, and that the risk of coexisting DU or GU increases with an increase in grade of AG of the antrum but decreases with an increase in grade of AG of the gastric body.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARD W. I., MARKS I. N. The relationship between the acid output of the stomach following "maximal" histamine stimulation and the parietal cell mass. Clin Sci. 1960 Feb;19:147–163. [PubMed] [Google Scholar]
- Cheli R., Giacosa A. Duodenal ulcer and chronic gastritis. Endoscopy. 1986 Jul;18(4):125–126. doi: 10.1055/s-2007-1018350. [DOI] [PubMed] [Google Scholar]
- Cheng F. C., Lam S. K., Ong G. B. Maximum acid output to graded doses of pentagastrin and its relation to parietal cell mass in Chinese patients with duodenal ulcer. Gut. 1977 Oct;18(10):827–832. doi: 10.1136/gut.18.10.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earlam R. J., Amerigo J., Kakavoulis T., Pollock D. J. Histological appearances of oesophagus, antrum and duodenum and their correlation with symptoms in patients with a duodenal ulcer. Gut. 1985 Jan;26(1):95–100. doi: 10.1136/gut.26.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear M. W., Truelove S. C., Whitehead R. Gastric ulcer and gastritis. Gut. 1971 Aug;12(8):639–645. doi: 10.1136/gut.12.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovinen E., Kekki M., Kuikka S. A theory to the stochastic dynamic model building for chronic progressive disease processes with an application to chronic gastritis. J Theor Biol. 1976 Mar;57(1):131–152. doi: 10.1016/s0022-5193(76)80009-4. [DOI] [PubMed] [Google Scholar]
- Hui W. M., Lam S. K., Ho J., Ng M. M., Lui I., Lai C. L., Lok A. S., Lau W. Y., Poon G. P., Choi S. Chronic antral gastritis in duodenal ulcer. Natural history and treatment with prostaglandin E1. Gastroenterology. 1986 Nov;91(5):1095–1101. doi: 10.1016/s0016-5085(86)80003-8. [DOI] [PubMed] [Google Scholar]
- Ippoliti A., Walsh J. Newer concepts in the pathogenesis of peptic ulcer disease. Surg Clin North Am. 1976 Dec;56(6):1479–1490. doi: 10.1016/s0039-6109(16)41100-x. [DOI] [PubMed] [Google Scholar]
- Kekki M., Sipponen P., Siurala M. Progression of antral and body gastritis in patients with active and healed duodenal ulcer and duodenitis. Scand J Gastroenterol. 1984 May;19(3):382–388. [PubMed] [Google Scholar]
- Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology. 1972 Oct;63(4):584–592. [PubMed] [Google Scholar]
- Maaroos H. I., Salupere V., Uibo R., Kekki M., Sipponen P. Seven-year follow-up study of chronic gastritis in gastric ulcer patients. Scand J Gastroenterol. 1985 Mar;20(2):198–204. doi: 10.3109/00365528509089657. [DOI] [PubMed] [Google Scholar]
- Mackay I. R., Hislop I. G. Chronic gastritis and gastric ulcer. Gut. 1966 Jun;7(3):228–233. doi: 10.1136/gut.7.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle D. D., Taylor K. B., Truelove S. C., Whitehead R. Gastritis duodenitis, and circulating levels of gastrin in duodenal ulcer before and after vagotomy. Gut. 1976 Sep;17(9):719–728. doi: 10.1136/gut.17.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister H., Holubarsch C., Haferkamp O., Schlag P., Herfarth C. Gastritis, intestinal metaplasia and dysplasia versus benign ulcer in stomach and duodenum and gastric carcinoma -- a histotopographical study. Pathol Res Pract. 1979 Jan;164(3):259–269. doi: 10.1016/S0344-0338(79)80048-5. [DOI] [PubMed] [Google Scholar]
- Moore S. C., Malagelada J. R., Shorter R. G., Zinsmeister A. R. Interrelationships among gastric mucosal morphology, secretion, and motility in peptic ulcer disease. Dig Dis Sci. 1986 Jul;31(7):673–684. doi: 10.1007/BF01296443. [DOI] [PubMed] [Google Scholar]
- Nielsen H. O., Muñoz J. D., Kronborg O., Andersen D. The antrum in duodenal ulcer patients. Relationship between antrum size, nerve of Latarjet, gastrin cell quantity, and gastric acid secretion. Scand J Gastroenterol. 1981;16(4):491–495. [PubMed] [Google Scholar]
- Oi M., Ito Y., Kumagai F., Yoshida K., Tanaka Y., Yoshikawa K., Miho O., Kijima M. A possible dual control mechanism in the origin of peptic ulcer. A study on ulcer location as affected by mucosa and musculature. Gastroenterology. 1969 Sep;57(3):280–293. [PubMed] [Google Scholar]
- Piper D. W., Greig M., Coupland G. A., Hobbin E., Shinners J. Factors relevant to the prognosis of chronic gastric ulcer. Gut. 1975 Sep;16(9):714–718. doi: 10.1136/gut.16.9.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samloff I. M., Stemmermann G. N., Heilbrun L. K., Nomura A. Elevated serum pepsinogen I and II levels differ as risk factors for duodenal ulcer and gastric ulcer. Gastroenterology. 1986 Mar;90(3):570–576. doi: 10.1016/0016-5085(86)91110-8. [DOI] [PubMed] [Google Scholar]
- Schrager J., Spink R., Mitra S. The antrum in patients with duodenal and gastric ulcers. Gut. 1967 Oct;8(5):497–508. doi: 10.1136/gut.8.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siurala M., Isokoski M., Varis K., Kekki M. Prevalence of gastritis in a rural population. Bioptic study of subjects selected at random. Scand J Gastroenterol. 1968;3(2):211–223. doi: 10.3109/00365526809180125. [DOI] [PubMed] [Google Scholar]
- Siurala M., Varis K., Kekki M. New aspects on epidemiology, genetics, and dynamics of chronic gastritis. Front Gastrointest Res. 1980;6:148–166. doi: 10.1159/000403329. [DOI] [PubMed] [Google Scholar]
- Stadelmann O., Elster K., Stolte M., Miederer S. E., Deyhle P., Demling L., Siegenthaler W. The peptic gastric ulcer--histotopographic and functional investigations. Scand J Gastroenterol. 1971;6(7):613–623. doi: 10.3109/00365527109181143. [DOI] [PubMed] [Google Scholar]
- Stemmermann G., Haenszel W., Locke F. Epidemiologic pathology of gastric ulcer and gastric carcinoma among Japanese in Hawaii. J Natl Cancer Inst. 1977 Jan;58(1):13–20. doi: 10.1093/jnci/58.1.13. [DOI] [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Trier J. S. Morphology of the gastric mucosa in patients with ulcer diseases. Am J Dig Dis. 1976 Feb;21(2):138–140. doi: 10.1007/BF01072059. [DOI] [PubMed] [Google Scholar]
- Varis K., Ihamäki T., Härkönen M., Samloff I. M., Siurala M. Gastric morphology, function, and immunology in first-degree relatives of probands with pernicious anemia and controls. Scand J Gastroenterol. 1979;14(2):129–139. doi: 10.3109/00365527909179858. [DOI] [PubMed] [Google Scholar]
- Whitehead R., Truelove S. C., Gear M. W. The histological diagnosis of chronic gastritis in fibreoptic gastroscope biopsy specimens. J Clin Pathol. 1972 Jan;25(1):1–11. doi: 10.1136/jcp.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


