Abstract
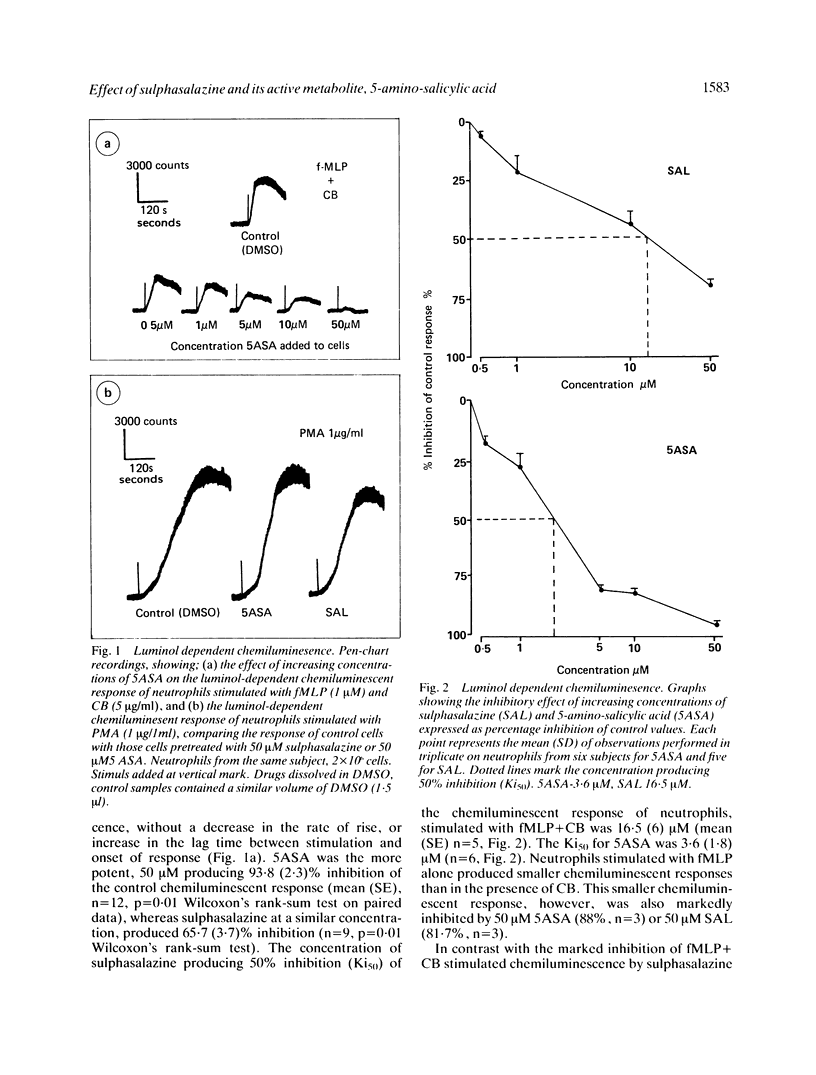
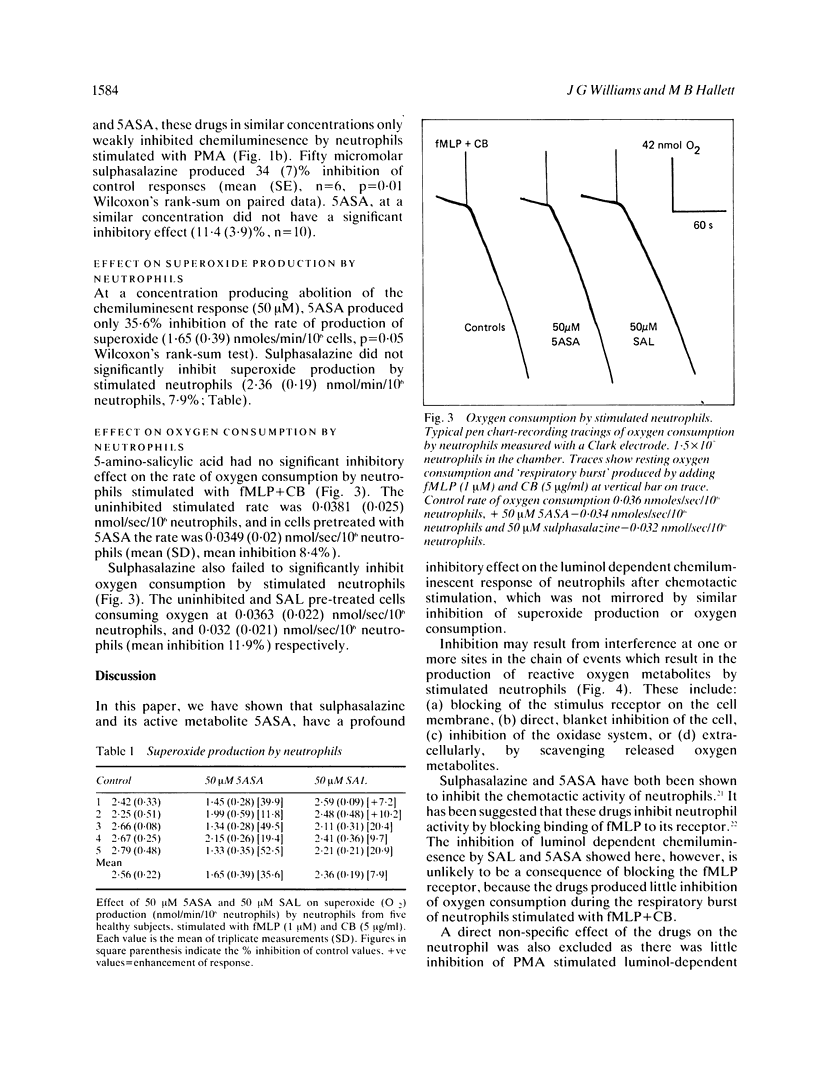
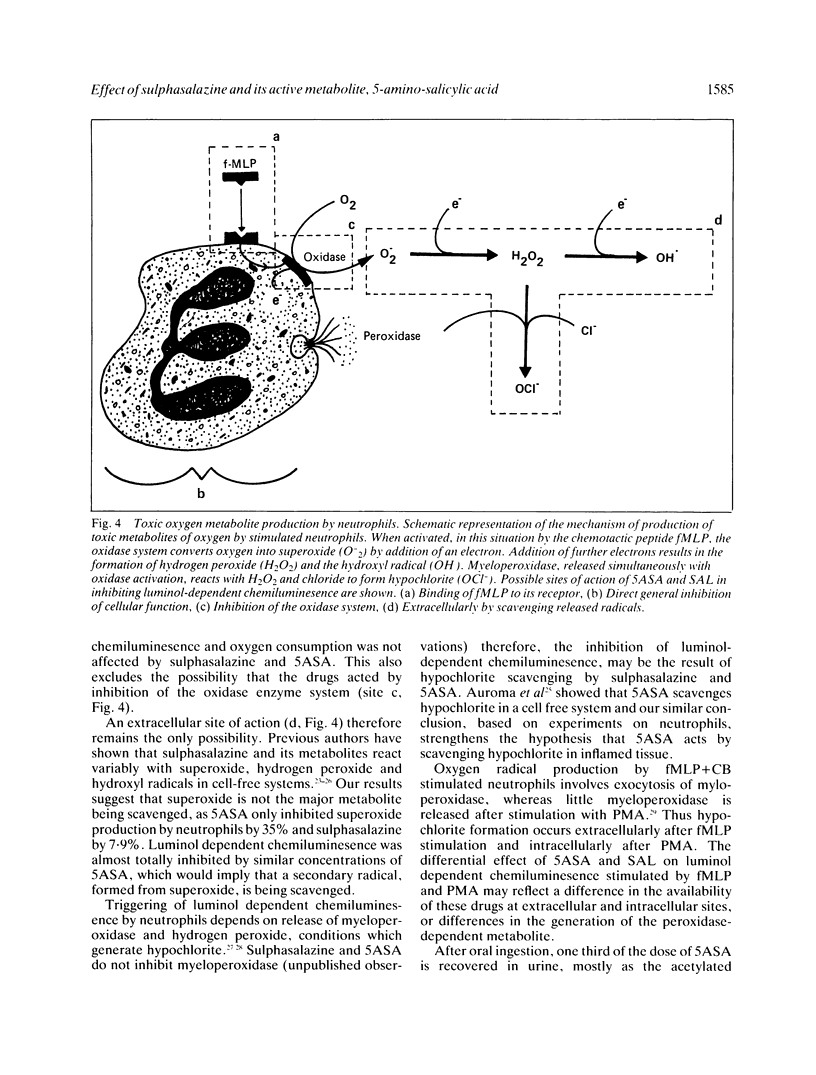
The possibility that the mode of action of sulphasalazine and its active metabolite 5-amino-salicylic acid (5ASA) involves modification of toxic oxygen metabolite production by neutrophils has been investigated by measuring the effect of these drugs on luminol-dependent chemiluminescence, superoxide release and oxygen consumption by stimulated neutrophils in vitro. 5ASA, and to a lesser extent sulphasalazine, had profound inhibitory effects on the luminol dependent chemiluminescent response of neutrophils stimulated with formyl-methionyl-leucyl-phenylalanine (1 microM) + cytochalasin B (5 micrograms/ml). A concentration of 50 microM 5ASA or sulphasalazine produced 93.8 (2.3)% and 65.7 (3.7)% inhibition of control responses respectively. The concentration of 5ASA and sulphasalazine producing 50% inhibition of chemiluminescence were 3.6 (1.8) microM and 16.5 (6) microM respectively. Both drugs had little effect on the chemiluminescent response of neutrophils stimulated with phorbol myristate acetate (1 microgram/ml), producing only 11.4 (3.9)% and 34 (7)% inhibition respectively, at a concentration of 50 microM. Superoxide release from fMLP + CB stimulated neutrophils was also inhibited slightly by 5ASA (50 microM) by 35.6% and by sulphasalazine (50 microM) by 7.9%. Similarly, there was little inhibition in the rate of oxygen consumption by fMLP + CB stimulated neutrophils by either 5ASA or sulphasalazine at concentrations which produced near total abolition of luminol dependent chemiluminescence. These results show that sulphasalazine and 5ASA inhibit the reaction of toxic metabolites produced by stimulated neutrophils with luminol, without inhibition of the oxidase system producing these metabolites. The site of action of these drugs on neutrophils in vitro is thus extracellular, by scavenging a released metabolite, probably hypochlorite. This has important implications for their mode of action in vivo in inflammatory bowel disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnfelt-Rønne I., Nielsen O. H. The antiinflammatory moiety of sulfasalazine, 5-aminosalicylic acid, is a radical scavenger. Agents Actions. 1987 Jun;21(1-2):191–194. doi: 10.1007/BF01974941. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Wasil M., Halliwell B., Hoey B. M., Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol. 1987 Nov 1;36(21):3739–3742. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Azad Khan A. K., Piris J., Truelove S. C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977 Oct 29;2(8044):892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B., Weeks I. Chemiluminescence as an analytical tool in cell biology and medicine. Methods Biochem Anal. 1985;31:317–416. doi: 10.1002/9780470110522.ch7. [DOI] [PubMed] [Google Scholar]
- Cooke E., Hallett M. B. The role of C-kinase in the physiological activation of the neutrophil oxidase. Evidence from using pharmacological manipulation of C-kinase activity in intact cells. Biochem J. 1985 Dec 1;232(2):323–327. doi: 10.1042/bj2320323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1983 Feb;39(2):736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Dew M. J., Hughes P. J., Lee M. G., Evans B. K., Rhodes J. An oral preparation to release drugs in the human colon. Br J Clin Pharmacol. 1982 Sep;14(3):405–408. doi: 10.1111/j.1365-2125.1982.tb01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull B. J., Salata K., Van Langenhove A., Goldman P. 5-Aminosalicylate: oxidation by activated leukocytes and protection of cultured cells from oxidative damage. Biochem Pharmacol. 1987 Aug 1;36(15):2467–2472. doi: 10.1016/0006-2952(87)90518-1. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Two distinct mechanisms for stimulation of oxygen-radical production by polymorphonuclear leucocytes. Biochem J. 1983 Nov 15;216(2):459–465. doi: 10.1042/bj2160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klotz U., Maier K. E. Pharmacology and pharmacokinetics of 5-aminosalicylic acid. Dig Dis Sci. 1987 Dec;32(12 Suppl):46S–50S. doi: 10.1007/BF01312463. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987 Feb;28(2):190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. Distribution studies of salicylazosulfapyridine and its metabolites. Gastroenterology. 1973 Feb;64(2):240–245. [PubMed] [Google Scholar]
- Rhodes J. M., Bartholomew T. C., Jewell D. P. Inhibition of leucocyte motility by drugs used in ulcerative colitis. Gut. 1981 Aug;22(8):642–647. doi: 10.1136/gut.22.8.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Stenson W. F., Mehta J., Spilberg I. Sulfasalazine inhibition of binding of N-formyl-methionyl-leucyl-phenylalanine (FMLP) to its receptor on human neutrophils. Biochem Pharmacol. 1984 Feb 1;33(3):407–412. doi: 10.1016/0006-2952(84)90233-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- van Hees P. A., Bakker J. H., van Tongeren J. H. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut. 1980 Jul;21(7):632–635. doi: 10.1136/gut.21.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


