Abstract
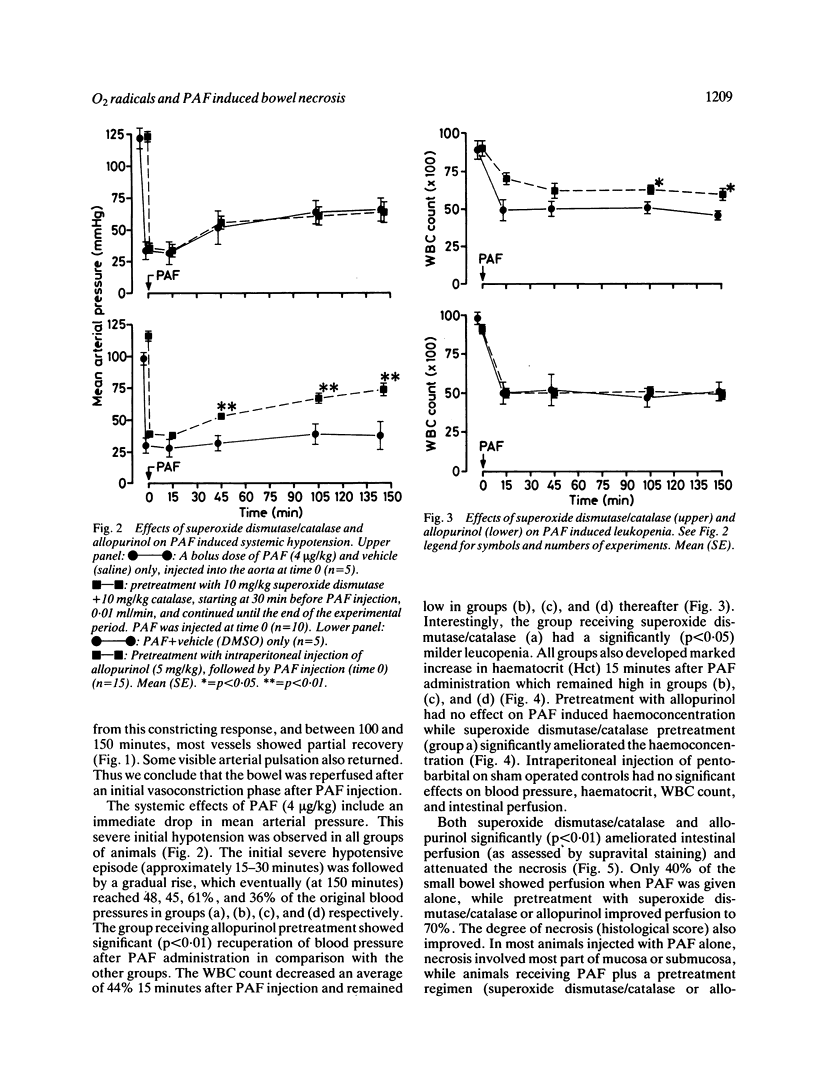
The mechanism of tissue and cell injury in ischaemic bowel necrosis is unclear. The present study investigated the role of oxygen derived free radicals in the development of bowel necrosis using injections of platelet activating factor (PAF) into the mesenteric vasculature. Animals were pretreated with allopurinol or superoxide dismutase together with catalase, before administration of PAF. Superoxide dismutase/catalase markedly improved the PAF-induced lesions, indicating that most of the intestinal damage after PAF injection is because of the release of oxygen radicals. The major source of oxygen radicals is xanthine oxidase, as allopurinol ameliorated small bowel lesions. Pretreatment with allopurinol produced a significant (p less than 0.01) preventive effect on PAF induced hypotension. In contrast, superoxide dismutase/catalase did not alter PAF induced hypotension. Superoxide dismutase/catalase pretreatment improved PAF induced haemoconcentration and leucopenia, while allopurinol showed no effect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atalla S. L., Toledo-Pereyra L. H., MacKenzie G. H., Cederna J. P. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985 Dec;40(6):584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Auscher C., Amory N., Pasquier C., Delbarre F. Localization of xanthine oxidase acitivty in hepatic tissue. A new histochemical method. Adv Exp Med Biol. 1977;76A:605–609. [PubMed] [Google Scholar]
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Dalsing M. C., Grosfeld J. L., Shiffler M. A., Vane D. W., Hull M., Baehner R. L., Weber T. R. Superoxide dismutase: a cellular protective enzyme in bowel ischemia. J Surg Res. 1983 Jun;34(6):589–596. doi: 10.1016/0022-4804(83)90115-4. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983 Jul;112(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Gonzalez-Crussi F., Arroyave J. L. Platelet-activating factor-induced ischemic bowel necrosis. An investigation of secondary mediators in its pathogenesis. Am J Pathol. 1986 Feb;122(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., Gonzalez-Crussi F., Arroyave J. L. Release of leukotriene C4 by isolated, perfused rat small intestine in response to platelet-activating factor. J Clin Invest. 1986 Jul;78(1):108–114. doi: 10.1172/JCI112538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., González-Crussi F., Arroyave J. L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987 Nov;1(5):403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- Jackson C. V., Mickelson J. K., Pope T. K., Rao P. S., Lucchesi B. R. O2 free radical-mediated myocardial and vascular dysfunction. Am J Physiol. 1986 Dec;251(6 Pt 2):H1225–H1231. doi: 10.1152/ajpheart.1986.251.6.H1225. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Kim M. S., Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol. 1987 Feb;252(2 Pt 2):H252–H257. doi: 10.1152/ajpheart.1987.252.2.H252. [DOI] [PubMed] [Google Scholar]
- Koyama I., Bulkley G. B., Williams G. M., Im M. J. The role of oxygen free radicals in mediating the reperfusion injury of cold-preserved ischemic kidneys. Transplantation. 1985 Dec;40(6):590–595. doi: 10.1097/00007890-198512000-00003. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Demopoulos C. A., Pinckard R. N. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J Immunol. 1980 Jun;124(6):2919–2924. [PubMed] [Google Scholar]
- Ouriel K., Smedira N. G., Ricotta J. J. Protection of the kidney after temporary ischemia: free radical scavengers. J Vasc Surg. 1985 Jan;2(1):49–53. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Muhl E., Sellin D., Younes M., Schildberg F. W., Haglund U. Posthypotensive generation of superoxide free radicals--possible role in the pathogenesis of the intestinal mucosal damage. Acta Chir Scand. 1984;150(4):301–309. [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]



