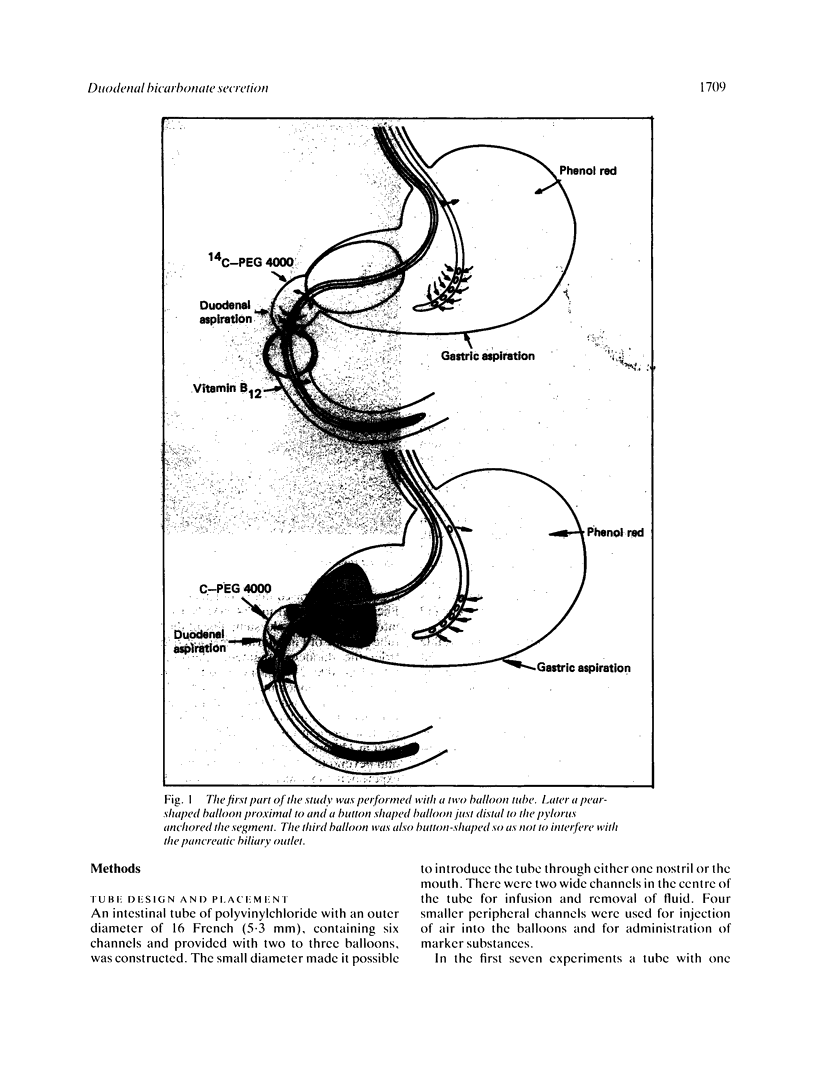
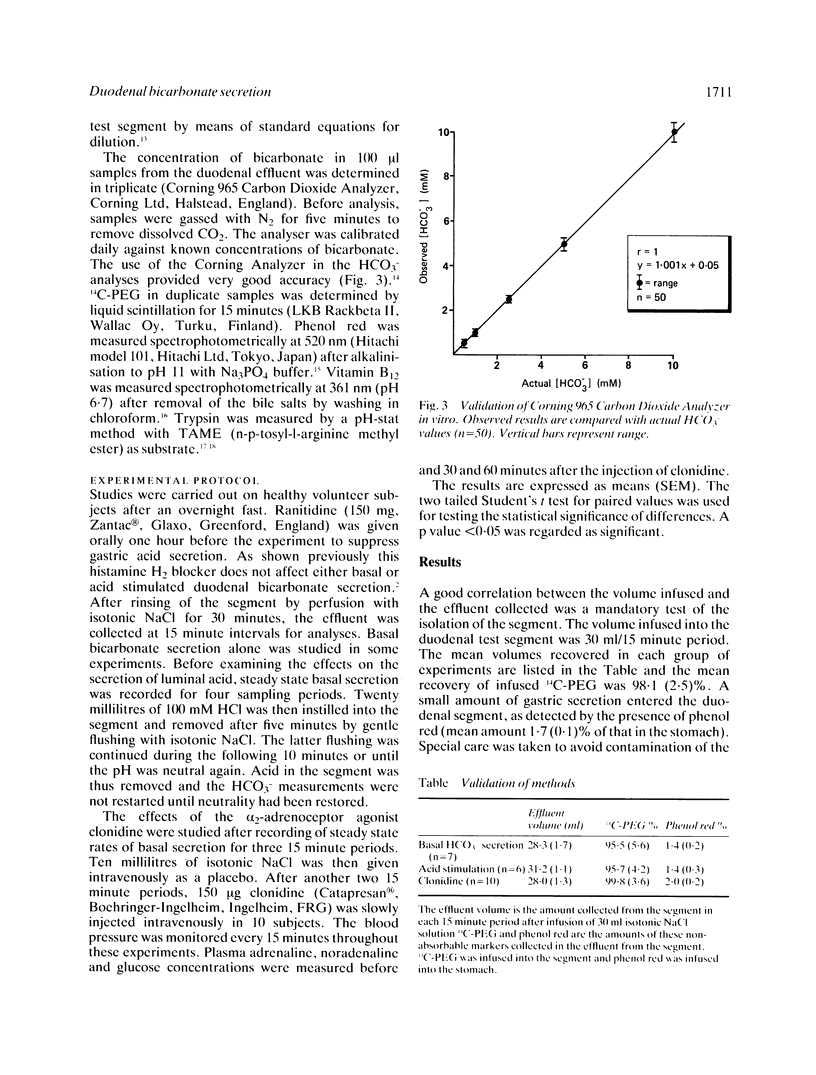
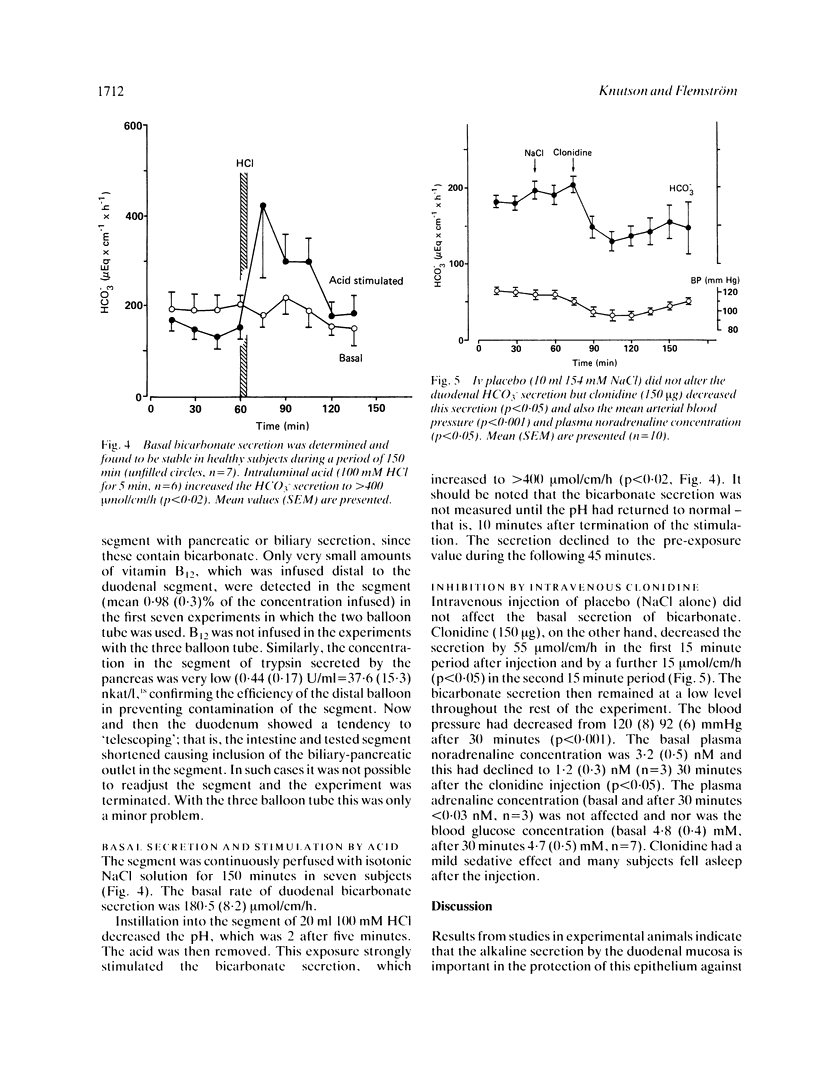
Abstract
A multi-channel small diameter tube was used to study the secretion of bicarbonate by 3 cm long segments of the proximal duodenum isolated between balloons. The tube had an outer diameter of 5.3 mm and two central and four smaller, peripheral channels. Measurements of infused phenol red, 14C-PEG and vitamin B12 and of trypsin activity were performed to rule out contamination of the perfusate by gastric and pancreatic secretions. Basal secretion of bicarbonate by the duodenal mucosa in healthy subjects varied between 135 and 220 mumol/cm of intestine per hour. Perfusion of the lumen with acid (100 mM HCl for five minutes) increased the secretion to greater than 400 mumol/cm/h and the alpha 2-adrenoreceptor agonist clonidine (150 micrograms iv) decreased the HCO3- secretion by 70 mumol/cm/h. Clonidine simultaneously reduced the mean arterial blood pressure and plasma noradrenaline concentration, but did not affect the plasma glucose or adrenaline concentration. Duodenal bicarbonate secretion is important in the protection of this mucosa against acid discharged from the stomach. Increased sympathetic activity may, by inhibiting the bicarbonate secretion, decrease the protection in proximal duodenum in man and facilitate ulceration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekker C., Andersen D., Kronborg O., Rørbaek Madsen P. E., Johansen T., Christiansen L. Plasma catecholamine and serum gastrin concentrations during sham feeding. Life Sci. 1983 Jan 17;32(3):257–262. doi: 10.1016/0024-3205(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Brandsborg O., Brandsborg M., Løvgreen N. A., Christensen N. J. Increased plasma noradrenaline and serum gastrin in patients with duodenal ulcer. Eur J Clin Invest. 1978 Feb;8(1):11–14. doi: 10.1111/j.1365-2362.1978.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Cross T. A. A Solid State Nuclear Magnetic Resonance Approach for Determining the Structure of Gramicidin a without Model Fitting. Biophys J. 1986 Jan;49(1):124–126. doi: 10.1016/S0006-3495(86)83620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin T., Rosenthal L., McArthur K., Anderson D., Dharmsathaphorn K. Clonidine and lidamidine (WHR-1142) stimulate sodium and chloride absorption in the rabbit intestine. Gastroenterology. 1982 Jun;82(6):1352–1358. [PubMed] [Google Scholar]
- Forssell H., Olbe L. Effect of fundic distension on gastric bicarbonate secretion in man. Scand J Gastroenterol. 1987 Jun;22(5):627–633. doi: 10.3109/00365528708991910. [DOI] [PubMed] [Google Scholar]
- Forssell H., Stenquist B., Olbe L. Vagal stimulation of human gastric bicarbonate secretion. Gastroenterology. 1985 Sep;89(3):581–586. doi: 10.1016/0016-5085(85)90454-8. [DOI] [PubMed] [Google Scholar]
- Fändriks L., Jönson C., Nylander O. Effects of splanchnic nerve stimulation and of clonidine on gastric and duodenal HCO3- secretion in the anaesthetized cat. Acta Physiol Scand. 1987 Jun;130(2):251–258. doi: 10.1111/j.1748-1716.1987.tb08134.x. [DOI] [PubMed] [Google Scholar]
- George J. D. New clinical method for measuring the rate of gastric emptying: the double sampling test meal. Gut. 1968 Apr;9(2):237–242. doi: 10.1136/gut.9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstam S. O., Flemström G., Nylander O. Bicarbonate secretion by the rabbit duodenum in vivo: effects of prostaglandins, vagal stimulation and some drugs. Acta Physiol Scand. 1987 Nov;131(3):377–385. doi: 10.1111/j.1748-1716.1987.tb08253.x. [DOI] [PubMed] [Google Scholar]
- Isenberg J. I., Selling J. A., Hogan D. L., Koss M. A. Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N Engl J Med. 1987 Feb 12;316(7):374–379. doi: 10.1056/NEJM198702123160704. [DOI] [PubMed] [Google Scholar]
- Jönson C., Fändriks L. Bleeding inhibits vagally induced duodenal HCO3- secretion via activation of the splanchnic nerves in anaesthetized rats. Acta Physiol Scand. 1987 Jun;130(2):259–264. doi: 10.1111/j.1748-1716.1987.tb08136.x. [DOI] [PubMed] [Google Scholar]
- Jönson C., Tunbäck-Hanson P., Fändriks L. Splanchnic nerve activation inhibits the increase in duodenal HCO3- secretion induced by luminal acidification in the rat. Gastroenterology. 1989 Jan;96(1):45–49. doi: 10.1016/0016-5085(89)90762-2. [DOI] [PubMed] [Google Scholar]
- Knutson L., Flemström G., Gustavsson S., Jedstedt G., Lönnerholm G. HCO3- secretion in rat duodenum after treatment with omeprazole and ranitidine. Scand J Gastroenterol. 1987 Jan;22(1):87–90. doi: 10.3109/00365528708991862. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Kwiecień N., Obtułowicz W., Thor P., Konturek J. W., Popiela T., Oleksy J. Vagal cholinergic control of gastric alkaline secretion in normal subjects and duodenal ulcer patients. Gut. 1987 Jun;28(6):739–744. doi: 10.1136/gut.28.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. E. Non-labelled vitamin B12 as a dilution indicator in gastrointestinal research. Scand J Clin Lab Invest. 1966;18(5):535–539. doi: 10.3109/00365516609103916. [DOI] [PubMed] [Google Scholar]
- Nylander O., Flemström G. Effects of alpha-adrenoceptor agonists and antagonists on duodenal surface epithelial HCO3-secretion in the rat in vivo. Acta Physiol Scand. 1986 Mar;126(3):433–441. doi: 10.1111/j.1748-1716.1986.tb07838.x. [DOI] [PubMed] [Google Scholar]
- PELOT D., GROSSMAN M. I. Distribution and fate of pancreatic enzymes in small intestine of the rat. Am J Physiol. 1962 Feb;202:285–288. doi: 10.1152/ajplegacy.1962.202.2.285. [DOI] [PubMed] [Google Scholar]
- Quigley E. M., Turnberg L. A. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology. 1987 Jun;92(6):1876–1884. doi: 10.1016/0016-5085(87)90619-6. [DOI] [PubMed] [Google Scholar]
- Rune S. J. Acid-base parameters of duodenal contents in man. Gastroenterology. 1972 Apr;62(4):533–539. [PubMed] [Google Scholar]
- Schmitt M. G., Jr, Wood C. M., Soergel K. H. A method for rapid placing of small intestinal perfusion tubes. Gut. 1974 Mar;15(3):227–228. doi: 10.1136/gut.15.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Hogan D. L., Isenberg J. I. A new method for quantitation of ion fluxes across in vivo human gastric mucosa: effect of aspirin, acetaminophen, ethanol, and hyperosmolar solutions. Gastroenterology. 1984 Jan;86(1):60–70. [PubMed] [Google Scholar]
- Wicks T., Clain D. A guide wire for rapid jejunal biopsies with the Crosby capsule. Gut. 1972 Jul;13(7):571–571. doi: 10.1136/gut.13.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]