Abstract
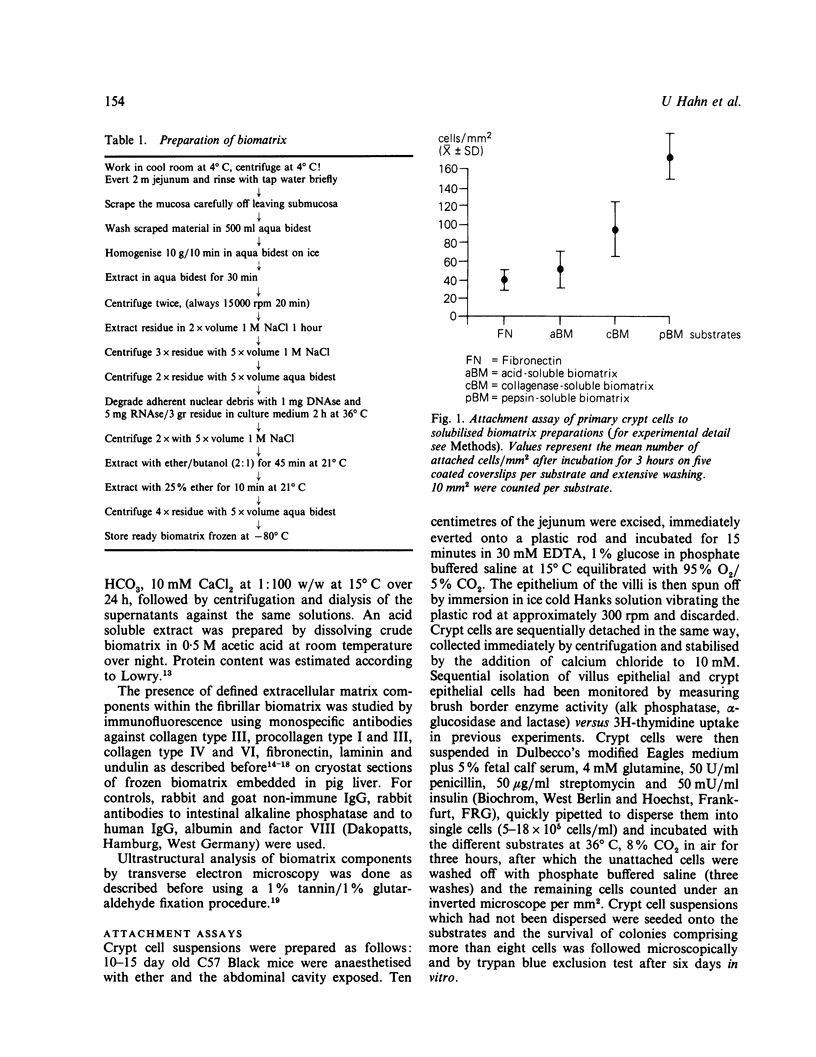
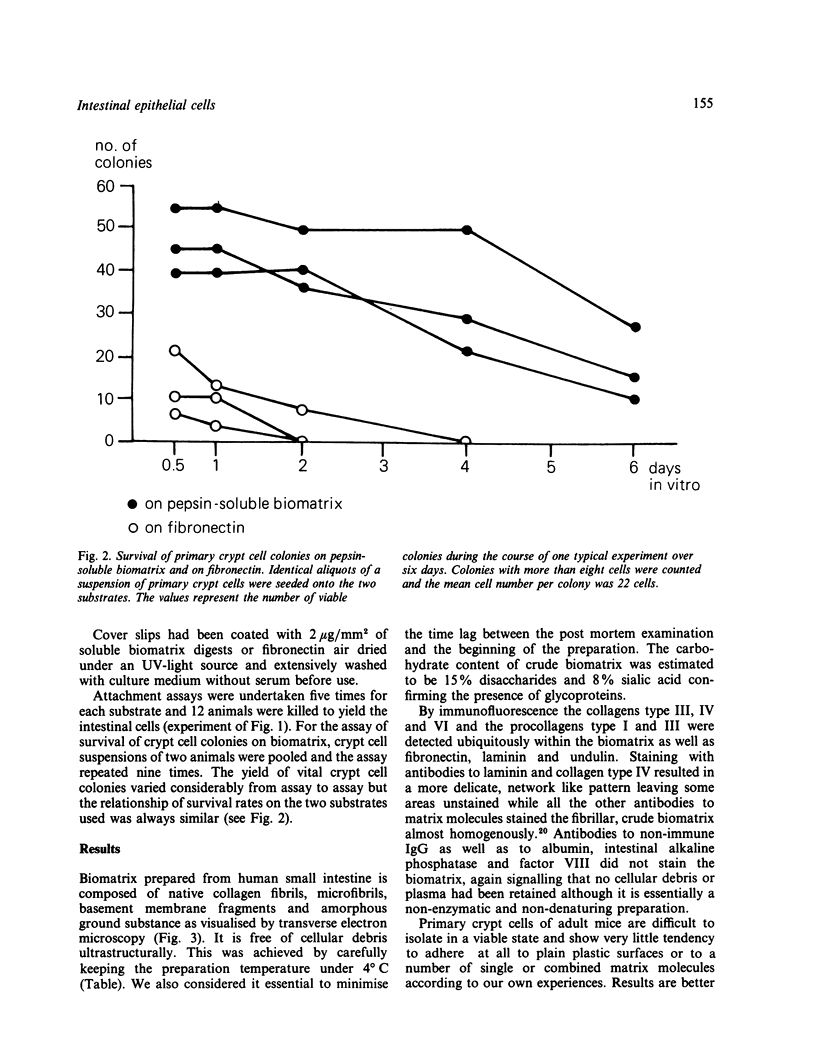
Primary intestinal epithelial cells have a very short lifespan in vitro when cultured free of mucosal elements. Support of the basal plasma membrane by a more natural substrate may thus enhance the initiation of primary cell cultures. A cell free biomatrix consisting of native interstitial collagens, basement membrane fragments and microfibrils was extracted from the lamina propria of human intestinal mucosa. Immunofluorescence revealed the presence of collagens type III, IV, and VI and procollagens type I and III as well as fibronectin, laminin and undulin. Primary crypt cells of suckling mice displayed a significantly increased affinity to pepsin and collagenase solubilised intestinal biomatrix when compared with plastic and fibronectin. Colonies of primary crypt cells survived for up to four days and longer on pepsin solubilised biomatrix but only for 48 hours on fibronectin. The intestinal biomatrix preparation has proved to be a useful substrate for the initiation and prolongation of primary intestinal cell cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burtin P., Chavanel G., Foidart J. M., Martin E. Antigens of the basement membrane and the peritumoral stroma in human colonic adenocarcinomas: an immunofluorescence study. Int J Cancer. 1982 Jul 15;30(1):13–20. doi: 10.1002/ijc.2910300104. [DOI] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin G. R., Kleinman H. K., Terranova V. P., Ledbetter S., Hassell J. R. The regulation of basement membrane formation and cell-matrix interactions by defined supramolecular complexes. Ciba Found Symp. 1984;108:197–212. doi: 10.1002/9780470720899.ch13. [DOI] [PubMed] [Google Scholar]
- Merker H. J., Barrach H. J. The morphology of basement membrane formation. Eur J Cell Biol. 1981 Dec;26(1):111–120. [PubMed] [Google Scholar]
- Négrel R., Rampal P., Nano J. L., Cavenel C., Ailhaud G. Establishment and characterization of an epithelial intestinal cell line from rat fetus. Exp Cell Res. 1983 Feb;143(2):427–437. doi: 10.1016/0014-4827(83)90069-1. [DOI] [PubMed] [Google Scholar]
- Ponce P., Cordero J., Rojkind M. A noncollagenous matrix for attachment of rat hepatocytes in culture. Hepatology. 1981 May-Jun;1(3):204–210. doi: 10.1002/hep.1840010303. [DOI] [PubMed] [Google Scholar]
- Quaroni A. Development of fetal rat intestine in organ and monolayer culture. J Cell Biol. 1985 May;100(5):1611–1622. doi: 10.1083/jcb.100.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Vargas L., Hahn E., Kalbfleisch H., Bruguera M., Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979 Dec;9(6):451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Becker J., Boehm H., Hahn E. G. Immunofluorescent localization of type-V collagen as a fibrillar component of the interstitial connective tissue of human oral mucosa, artery and liver. Cell Tissue Res. 1986;243(3):535–543. doi: 10.1007/BF00218060. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Besser M., Schwarting R., Hahn E. G. Radioimmunoassay for the carboxy-terminal cross-linking domain of type IV (basement membrane) procollagen in body fluids. Characterization and application to collagen type IV metabolism in fibrotic liver disease. J Clin Invest. 1986 Jul;78(1):241–248. doi: 10.1172/JCI112557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Rühlmann T., Hahn E. G. Radioimmunoassay for human type VI collagen and its application to tissue and body fluids. Anal Biochem. 1985 Aug 15;149(1):238–247. doi: 10.1016/0003-2697(85)90501-9. [DOI] [PubMed] [Google Scholar]
- Timpl R., Johansson S., van Delden V., Oberbäumer I., Hök M. Characterization of protease-resistant fragments of laminin mediating attachment and spreading of rat hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8922–8927. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



